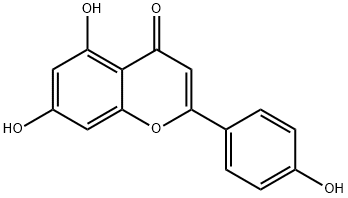
Apigenin
- CAS No.
- 520-36-5
- Chemical Name:
- Apigenin
- Synonyms
- CHAMOMILE;5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one;apigenol;apigenine;5,7-dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone;Apig;75580;75590;Apegenin;UCCF 031
- CBNumber:
- CB1384541
- Molecular Formula:
- C15H10O5
- Molecular Weight:
- 270.24
- MDL Number:
- MFCD00006831
- MOL File:
- 520-36-5.mol
- MSDS File:
- SDS
| Melting point | >300 °C (lit.) |
|---|---|
| Boiling point | 333.35°C (rough estimate) |
| Density | 1.2319 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: 27 mg/mL |
| form | powder |
| pka | 6.53±0.40(Predicted) |
| color | yellow |
| Merck | 14,730 |
| BRN | 262620 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| InChI | InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H |
| InChIKey | KZNIFHPLKGYRTM-UHFFFAOYSA-N |
| SMILES | C1(C2=CC=C(O)C=C2)OC2=CC(O)=CC(O)=C2C(=O)C=1 |
| LogP | 3.020 |
| CAS DataBase Reference | 520-36-5(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | chamomile |
| FDA UNII | 7V515PI7F6 |
| NCI Drug Dictionary | chamomile |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a-P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LK9276000 | |||||||||
| F | 10 | |||||||||
| HS Code | 29329985 | |||||||||
| NFPA 704 |
|
Apigenin price More Price(83)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHL89159 | Apigenin phyproof? Reference Substance | 520-36-5 | 25mg | $344 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1040683 | Apigenin United States Pharmacopeia (USP) Reference Standard | 520-36-5 | 30mg | $527 | 2024-03-01 | Buy |
| Sigma-Aldrich | 01760595 | Apigenin primary pharmaceutical reference standard | 520-36-5 | 10mg | $463 | 2023-06-20 | Buy |
| TCI Chemical | A1514 | Apigenin >98.0%(HPLC) | 520-36-5 | 100mg | $151 | 2024-03-01 | Buy |
| Alfa Aesar | L15041 | 4',5,7-Trihydroxyflavone, 97% | 520-36-5 | 25mg | $59.2 | 2024-03-01 | Buy |
Apigenin Chemical Properties,Uses,Production
Description
Apigenin(520-36-5) is one of the most widespread flavonoids in plants and formally belongs to the flavone sub-class. Of all the flavonoids, apigenin is one of the most widely distributed in the plant kingdom, and one of the most studied phenolics. Apigenin is present principally as glycosylated in significant amount in vegetables (parsley, celery, onions) fruits (oranges), herbs (chamomile, thyme, oregano, basil), and plant-based beverages (tea, beer, and wine). Plants belonging to the Asteraceae, such as those belonging to Artemisia, Achillea, Matricaria, and Tanacetum genera, are the main sources of this compound.
Chemical Properties
Pale Yellow Crystalline Solid
Uses
Apigenin has been shown to possess antibacterial, antiviral, antifungal, and antiparasitic activities. Although it can’t stop all types of bacteria on its own, it can be combined with other antibiotics to increase their effects.
Uses
Apigenin is a promising reagent for cancer therapy. Apigenin appears to have the potential to be developed either as a dietary supplement or as an adjuvant chemotherapeutic agent for cancer therapy.
Uses
Apigenin is an active antioxidant, anti-inflammatory, anti-amyloidogenic, neuroprotective and cognitive enhancing substance with interesting potential in the treatment/prevention of Alzheimer's disease.
Preparation
4-hydroxybenzaldehyde (1.22 g, 9.97 mmol, 1.0 equiv) was added to asolution of 50% KOH (aq.) (6.72 g, 59.82 mmol, 6.0 equiv) and ethanol (3 mL) andstirred for 10 min. Then compound 9 (2.02g, 9.97 mmol, 1.0 equiv) was added to the reaction mixture and heated to 60 °C and stirred for 4 h. After cooled toroom temperature, the mixture was poured into ice water and acidified withconcentrated hydrochloric acid to pH = 3. Then the suspension was filtrated, washed and the residue was dried toafford Apigenin(2.43 g, 90%) as a red solid. 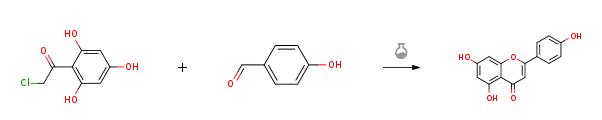
Definition
ChEBI: Apigenin is a trihydroxyflavone that is flavone substituted by hydroxy groups at positions 4', 5 and 7. It induces autophagy in leukaemia cells. It has a role as a metabolite and an antineoplastic agent. It is a conjugate acid of an apigenin-7-olate.
benefits
Apigenin has various beneficial health effects such as antioxidant, anti-inflammatory, and chemoprevention. Apigenin can downregulate the expression of IL-1β and TNF-α in LPS-stimulated mouse macrophages and human monocytes. preclinical studies have suggested that apigenin may improve outcomes in multiple health states, including anxiety, brain function, oxidative stress, inflammation, and hormonal regulation (testosterone, estrogen, and cortisol
Mechanism of action
Apigenin exerts anxiolytic effects at high doses by inhibiting NMDA receptors; it also has affinity to GABA-A receptors. Apigenin also exerts potent antioxidant activities by scavenging free radicals and upregulating glutathione levels; it also exerts anti-inflammatory effects. Apigenin is most studied for its potential anti-cancer properties.
Anticancer Research
It induces apoptosis by targeting leptin/leptin receptor pathway and by targeting caspase-dependent extrinsic pathway aswell as STAT3 signaling pathway in lung adenocarcinoma and BT-474 breast cancercells, respectively.
It shows antitumor activity against breastcancer MCF-7 cells and colon cancer HCT 116 cells and is a mediator of cancerchemoprevention and an inducer of autophagy. It can be used to treat colon canceras it induces apoptosis in colon cancer cells. It also increase melanogenesis in B16cells by activating the p38 MAPK pathway.
Safety
Apigenin is considered safe when consumed in normal amounts through a diet rich in fruits, vegetables, and herbs. However, supplement doses tend to deliver a significantly higher amount of apigenin than would be generally consumed via dietary means. Higher doses of apigenin can cause stomach discomfort, and people should cease using it immediately and consult medical professionals. At high doses, it can trigger muscle relaxation and sedation[1]. Allergic reactions can also occur as a response to chamomile tea or apigenin.
Source
Apigenin (4′,5,7-trihydroxyflavone) is one of the most widespread flavonoids in plants and formally belongs to the flavone sub-class. Plants belonging to the Asteraceae, such as those belonging to Artemisia, Achillea, Matricaria, and Tanacetum genera, are the main sources of this compound. However, species belonging to other families, such as the Lamiaceae, for instance, Sideritis and Teucrium, or species from the Fabaceae, such as Genista, showed the presence of apigenin in the aglycone form and/or its C- and O-glucosides, glucuronides, O-methyl ethers, and acetylated derivatives. In gymnosperms, apigenin derivatives are mostly present in dimeric forms, with apigenin residues variously coupled, e.g., with C?C linkage as in cupressuflavone and amentoflavone (I-8, II-8″ and I-3′, II-8″, respectively), or C—O linkage (I-4′, II-6″) as in hinokiflavone.
storage
+4°C
Purification Methods
The current method for the purification of apigenin is crude-solvent extraction method by using solvent in small quantity and also time saving. Apigenin that was isolated from Symphyotrichum novae-angliae was obtained in large quantity and directly from extract.
References
[1] Sanjeev Shukla, Sanjay Gupta (2010) Apigenin: a promising molecule for cancer prevention, 27, 962-978.
[2] https://en.wikipedia.org/wiki/Apigenin
[3] http://bodynutrition.org/apigenin/
References
[1] Bahare Salehi, et al. “The Therapeutic Potential of Apigenin.” Int J Mol Sci. 2019 Mar; 20(6): 1305.
Apigenin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Bonerge(Hunan) Lifescience Co., Ltd. | +86-731-82791134 +86-18801900056 | hank.h@bonerge.com | China | 30 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 2995 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Changsha Staherb Natural Ingredients Co., Ltd. | +86-0731-84213302 +8618374838656 | sales@staherb.cn | China | 1025 | 58 |
| Shaanxi LonierHerb Bio Technology Co Ltd | +86-86-+86-86-029-87551862 +8617702909819 | sales006@ingredients-lonier.com | China | 2596 | 58 |
| Hangzhou Zelixir Biotech Co., Ltd. | +8618867646786 | neal.chen@zelixir.com | China | 229 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 282 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
Related articles
- Apigenin: a promising natural compound with numerous health benefits
- Apigenin is a naturally occurring flavone that belongs to the class of compounds called flavonoids. It is a promising natural ....
- May 30,2023
- What is apigenin?
- Apigenin is a flavonoid of low toxicity and multiple beneficial bioactivities.
- Feb 26,2020
Related Qustion
- Q:Is Apigenin a dietary supplement?
- A:Yes, apigenin can be used as a dietary supplement in everyday life, in fact, it is found in fruits, vegetables, herbs and beve....
- Nov 23,2023
View Lastest Price from Apigenin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Apigenin
520-36-5
|
US $0.00-0.00 / kg | 0.1kg | 99% | 200 | Hangzhou Zelixir Biotech Co., Ltd. | |
 |
2024-04-24 | Apigenin
520-36-5
|
US $505.00-490.00 / kilograms | 100kilograms | 99% | 100tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-23 | Apigenin
520-36-5
|
US $10.00 / kg | 1kg | 95% | 95% 98%HPLC | Changsha Staherb Natural Ingredients Co., Ltd. |