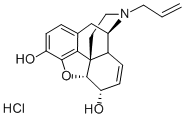
NALORPHINE HYDROCHLORIDE
- CAS No.
- 57-29-4
- Chemical Name:
- NALORPHINE HYDROCHLORIDE
- Synonyms
- NIH-10124;miromorfalil;Dea no. 9400;Nalorphine HCl;Einecs 200-321-8;nalinehydrochloride;Nalorphine chloride;allylmorphinehydrochloride;n-allyl-normorphinhydrochloride;n-allylnormorphinehydrochloride
- CBNumber:
- CB1501438
- Molecular Formula:
- C19H21NO3.ClH
- Molecular Weight:
- 347.84
- MDL Number:
- MFCD00055164
- MOL File:
- 57-29-4.mol
| Melting point | 260-263° |
|---|---|
| Flash point | 11 °C |
| storage temp. | 2-8°C |
| solubility | H2O: slightly soluble |
| form | solid |
| color | white |
| EWG's Food Scores | 1 |
| FDA UNII | 9FPE56Z2TW |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P301+P312+P330 |
| Hazard Codes | Xn,T,F |
| Risk Statements | 22-39/23/24/25-23/24/25-11 |
| Safety Statements | 36/37/39-45-36/37-16-7 |
| RIDADR | UN 1230 3/PG 2 |
| WGK Germany | 3 |
| RTECS | QC7710000 |
| HS Code | 2939190000 |
| Toxicity | LD50 s.c. in rats: 1460 mg/kg (Goldenthal) |
NALORPHINE HYDROCHLORIDE Chemical Properties,Uses,Production
Originator
Nalline,MSD,US,1952
Uses
Narcotic antagonist. Controlled substance.
Manufacturing Process
6 grams of normorphine, 2.7 grams of allyl bromide, 2.65 grams of sodium bicarbonate, and 75 cc of methanol were mixed together, and the resulting mixture was heated under reflux with stirring for a period of about 5 1/2 hours. The reaction mixture was evaporated to dryness in vacuo, the residual material was extracted with 60 cc of boiling chloroform, 0.5 gram of activated charcoal was added, and the resulting mixture was filtered through a layer of diatomaceous silica. The filter cake was washed with four 10 cc portions of boiling chloroform, and the chloroform filtrate and washings were combined and evaporated to dryness in vacuo. The residual material was triturated with 25 cc of anhydrous ether until crystalline, the ethereal mixture was cooled, maintained at a temperature of 3°C overnight, filtered, and the crystalline mixture was washed with three 10 cc portions of ice-cold ether. The resulting crystalline product was dried to give 6.0 grams of N-allylnormorphine, yield approximately 87% of theory, according to US Patent 2,891,954.
Therapeutic Function
Narcotic antagonist