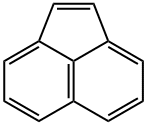
ACENAPHTHYLENE
- CAS No.
- 208-96-8
- Chemical Name:
- ACENAPHTHYLENE
- Synonyms
- ACENAPHTHALENE;Acenaphthylene Standard;Cyclopenta[de]naphthalene;Acenaphthylene, tech., 80%;DYPR0407;ACENAPHTHYLENE;6'-Dihydroxy-3';Acenaphthylene RS;ACENAPHTHYLENE 95%;Acenaphthylene >
- CBNumber:
- CB1665589
- Molecular Formula:
- C12H8
- Molecular Weight:
- 152.19
- MDL Number:
- MFCD00003806
- MOL File:
- 208-96-8.mol
- MSDS File:
- SDS
| Melting point | 78-82 °C(lit.) |
|---|---|
| Boiling point | 280 °C(lit.) |
| Density | 0.899 g/mL at 25 °C(lit.) |
| vapor pressure | 6.68 at 25 °C (gas saturation-HPLC/UV spectrophotometry, Sonnefeld et al., 1983) |
| refractive index | 1.6360 (estimate) |
| Flash point | 122°C |
| storage temp. | room temp |
| solubility | Soluble in ethanol, ether, and benzene (U.S. EPA, 1985) |
| color | Yellow |
| Specific Gravity | 0.899 |
| Water Solubility | 3.93mg/L(25 ºC) |
| BRN | 774092 |
| Henry's Law Constant | 1.14 at 25 °C (gas stripping-UV spectrophotometry, Warner et al., 1987) |
| Stability | Stable. Incompatible with oxidizing agents. |
| InChIKey | HXGDTGSAIMULJN-UHFFFAOYSA-N |
| CAS DataBase Reference | 208-96-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 1Z25C36811 |
| EPA Substance Registry System | Acenaphthylene (208-96-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P270-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N,F,T,Xi | |||||||||
| Risk Statements | 22-36/37/38-67-65-50/53-38-11-39/23/24/25-23/24/25-20 | |||||||||
| Safety Statements | 26-36/37/39-62-61-60-45-36/37-16-7-37/39-33-25-9 | |||||||||
| RIDADR | UN 1145 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AB1254000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29029090 | |||||||||
| Toxicity | LC50 (21-d) for Folsomia fimetaria 145 mg/kg (Sverdrup et al., 2002). | |||||||||
| NFPA 704 |
|
ACENAPHTHYLENE price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 48566 | Acenaphthylene analytical standard | 208-96-8 | 100mg | $87.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 416703 | Acenaphthylene 99% | 208-96-8 | 100mg | $160 | 2024-03-01 | Buy |
| Sigma-Aldrich | 92549 | Acenaphthylene certified reference material, TraceCERT | 208-96-8 | 100mg | $530 | 2022-05-15 | Buy |
| TCI Chemical | A0005 | Acenaphthylene >94.0%(GC) | 208-96-8 | 5g | $30 | 2024-03-01 | Buy |
| TCI Chemical | A0005 | Acenaphthylene >94.0%(GC) | 208-96-8 | 25g | $96 | 2024-03-01 | Buy |
ACENAPHTHYLENE Chemical Properties,Uses,Production
Description
Acenaphthylene is a PAH with three aromatic rings. It is an intermediate chemical for the manufacture of dyes, soaps, pigments, pharmaceuticals, insecticides, fungicides, herbicides, plant growth hormones, naphthalic acids, naphthalic anhydride (pigments), and acenaphthylene (resins) and is used to manufacture plastics. The largest emissions of PAHs result from incomplete combustion of organic materials during industrial processes and other human activities. These include (1) processing of coal, crude oil, and natural gas, including coking, coal conversion, and petroleum refining; (2) production of carbon blacks, creosote, coal tar, and bitumen; (3) aluminium, iron, and steel production in plants and foundries; (4) heating in power plants and residences and cooking; (5) combustion of refuse; (6) motor vehicle traffic; and (7) environmental tobacco smoke.
Chemical Properties
Acenaphthylene is a flaky yellow crystalline powder or solid.
Chemical Properties
Acenaphthylene is a polycyclic aromatic hydrocarbon (PAH) with three aromatic rings. It is used to manufacture plastics. Intermediate for dyes, soaps, pigments, pharmaceuticals, insec- ticide, fungicide, herbicide, and plant growth hormones. Intermediate for naphthalic acids, naphthalic anhydride (intermediate for pigments), and acenaphthylene (intermediate for res- ins). The largest emissions of PAH result from incomplete combustion of organic materials during industrial processes and other human activities. These include (a) processing of coal, crude oil, and natural gas, including coal; (b) coking, coal conversion, petroleum refi ning, and production of carbon blacks, creosote, coal-tar, and bitumen; (c) aluminium, iron, and steel production in plants and foundries; (d) heating in power plants and residences and cooking; (e) combustion of refuse; (f) motor vehicle traffi c; and (g) environmental tobacco smoke.
Physical properties
Colorless to white prisms or crystalline plates from alcohol with an odor similar to coal tar or aromatic hydrocarbons.
Uses
Polycyclic aromatic hydrocarbons as carcinogenic
Uses
Acenaphthylene may be used as an analytical reference standard for the determination of the analyte in water using ?gas chromatography-flame ionization detection (GC-FID).
Uses
Acenaphthylene has been used to investigate the photodimerization of acenaphthylene in micellar and hydrogel media.
Definition
ChEBI: A ortho- and peri-fused tricyclic hydrocarbon that occurs in coal tar.
Synthesis Reference(s)
Journal of the American Chemical Society, 69, p. 1388, 1947 DOI: 10.1021/ja01198a044
The Journal of Organic Chemistry, 61, p. 324, 1996 DOI: 10.1021/jo951613a
General Description
Colorless crystalline solid. Insoluble in water. Used in dye synthesis, insecticides, fungicides, and in the manufacture of plastics.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as ACENAPHTHYLENE, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.
Health Hazard
Acenapht hylene is i rritat i ng to t he sk i n a nd mucous membra nes of rabbits. Subc h ron ic oral doses of acenaphthylene caused adverse effects to the kidneys, liver, blood, reproductive system, and lungs of experimental animals. Prolonged period of inhalation at low doses caused pulmonary effects like bronchitis, pneumonia, and desquamation of the bronchial and alveolar epithelium in rats.
Health Hazard
Carcinogenic properties of this compound inanimals or humans are not known. Toxicitydata are not available.
Safety Profile
Moderately toxic by intraperitonealroute. Mutation data reported. When heated todecomposition it emits acrid smoke and irritating fumes.
Potential Exposure
PAHs are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons. Acenaphthylene is an aromatic hydrocarbon used in coal tar processing, as a dye intermediate; making insecticides, fungicides, plastics.
Source
Detected in groundwater at a former coal gasification plant in Seattle, WA at concentrations
ranging from nondetect (method detection limit 5 μg/L) to 250 μg/L (ASTR, 1995). Based
on laboratory analysis of 7 coal tar samples, acenaphthylene concentrations ranged from 260 to
18,000 ppm (EPRI, 1990). Lee et al. (1992a) equilibrated 8 coal tars with distilled water at 25 °C.
The maximum concentration of acenaphthylene observed in the aqueous phase was 0.5 mg/L.
Acenaphthylene was detected in asphalt fumes at an average concentration of 6.93 ng/m3 (Wang
et al., 2001).
Detected in a distilled water-soluble fraction of used motor oil at concentrations ranging from
4.5 to 4.6 μg/L (Chen et al., 1994).
Acenaphthylene was detected in a diesel-powered medium duty truck exhaust at an emission
rate of 70.1 μg/km (Schauer et al., 1999). Acenaphthylene was also detected in soot generated
from underventilated combustion of natural gas doped with toluene (3 mole %) (Tolocka and
Miller, 1995).
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 37.0 and 2,180 μg/km, respectively (Schauer et al., 2002).
Nine commercially available creosote samples contained acenaphthylene at concentrations
ranging from 7 to 3,700 mg/kg (Kohler et al., 2000).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rates of acenaphthylene were 18.6 mg/kg of pine burned, 10.8 mg/kg of oak burned, and 9.99
mg/kg of eucalyptus burned.
Environmental Fate
Biological. When acenaphthylene was statically incubated in the dark at 25 °C with yeast
extract and settled domestic wastewater inoculum, significant biodegradation with rapid
adaptation was observed. At concentrations of 5 and 10 mg/L, 100 and 94% biodegradation,
respectively, were observed after 7 d (Tabak et al., 1981). A Beijerinckia sp. and a mutant strain
were able to cooxidize acenaphthylene to the following metabolites: acenaphthenequinone and a
compound tentatively identified as 1,2-dihydroxyacenaphthylene. When acenaphthylene was
incubated with a mutant strain (Beijerinckia sp. strain B8/36) one metabolite formed which was
tentatively identified as cis-1,2-acenaphthenediol (Schocken and Gibson, 1984). This compound
also formed when acenaphthylene was deoxygenated by a recombinant strain of Pseudomonas
aeruginosa PAO1(pRE695) (Selifonov et al., 1996).
Bossert and Bartha (1986) reported that acenaphthylene in a Nixon sandy loam soil (1
g/kg) completely disappeared in <4 months. They concluded volatilization was more important
than biodegradation in the disappearance of acenaphthylene from soil.
Ozonation in water at 60 °C produced 1,8-naphthalene dialdehyde, 1,8-
naphthalene anhydride, 1,2-epoxyacenaphthylene, 1-naphthoic acid, and 1,8-naphthaldehydic acid
(Calvert and Pitts, 1966).
Shipping
UN3143 Dye intermediates, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous material, Hazard, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Dissolve acenaphthylene in warm redistilled MeOH, filter through a sintered glass funnel and cool to -78o to precipitate the material as yellow plates [Dainton et al. Trans Faraday Soc 56 1784 1960]. Alternatively it can be sublimed in vacuo. [Beilstein 5 H 625, 5 IV 2138.]
Incompatibilities
Keep away from ozone and strong oxidizing agents. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Product residues and sorbent media may be packaged in epoxy-lined drums, then destroyed by incineration, permanganate oxidation or microwave plasma treatment. The United States Environmental Protection Agency has investigated chemical precipitation for wastewater treatment
ACENAPHTHYLENE Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| AB PharmaTech,LLC | 323-480-4688 | United States | 989 | 55 | |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
View Lastest Price from ACENAPHTHYLENE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-25 | ACENAPHTHYLENE
208-96-8
|
US $8.00 / kg | 1kg | 99% | 20000 | Hebei Jingbo New Material Technology Co., Ltd | |
 |
2023-07-22 | Acenaphthylene
208-96-8
|
US $100.00 / kg | 1kg | 99% | 500t/month | Henan Bao Enluo International TradeCo.,LTD | |
 |
2023-04-13 | Acenaphthylene
208-96-8
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Guanlang Biotechnology Co,.LTD |
-

- ACENAPHTHYLENE
208-96-8
- US $8.00 / kg
- 99%
- Hebei Jingbo New Material Technology Co., Ltd
-

- Acenaphthylene
208-96-8
- US $100.00 / kg
- 99%
- Henan Bao Enluo International TradeCo.,LTD
-

- Acenaphthylene
208-96-8
- US $5.00-2.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD
208-96-8(ACENAPHTHYLENE)Related Search:
1of4