Zofenopril
- CAS No.
- 81872-10-8
- Chemical Name:
- Zofenopril
- Synonyms
- NAA;NAA(R);NAA(TM);ALPHA-NAA;Zofenopril;ROOTONE(R);FRUIT FAX(R);Zofenopril-d5;AKOS AU36-M588;RARECHEM AL BO 0198
- CBNumber:
- CB1679843
- Molecular Formula:
- C22H23NO4S2
- Molecular Weight:
- 429.55
- MDL Number:
- MFCD00870957
- MOL File:
- 81872-10-8.mol
| Melting point | 129-131.5 °C(lit.) |
|---|---|
| alpha | D25 -36.5° (c = 1 in methanol) |
| Boiling point | 646.3±55.0 °C(Predicted) |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 5 mg/mL (11.64 mM) |
| form | crystalline |
| pka | pK1:4.236 (25°C) |
| color | light yellow |
| CAS DataBase Reference | 81872-10-8(CAS DataBase Reference) |
| FDA UNII | 290ZY759PI |
| ATC code | C09AA15 |
SAFETY
Risk and Safety Statements
| Hazard Codes | Xn,Xi |
|---|---|
| Risk Statements | 22-37/38-41 |
| Safety Statements | 23-24/25 |
| WGK Germany | 3 |
| RTECS | QJ0875000 |
| HazardClass | IRRITANT |
Zofenopril price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ChemScene | CS-7911 | Zofenopril 98.81% | 81872-10-8 | 5mg | $192 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | RDL0007209 | ZOFENOPRIL-D5 95.00% | 81872-10-8 | 5MG | $502.66 | 2021-12-16 | Buy |
| CSNpharm | CSN21981 | Zofenopril | 81872-10-8 | 5mg | $163 | 2021-12-16 | Buy |
Zofenopril Chemical Properties,Uses,Production
Originator
Zofenopril Calcium,ZYF Pharm Chemical
Uses
Zofenopril-d5 is intended for use as an internal standard for the quantification of Zofenopril by GC- or LC-mass spectrometry.
Definition
ChEBI: A proline derivative that is 4-(phenylsulfanyl)-L-proline in which the amine proton is replaced by a (2S)-3-(benzoylsulfanyl)-2-methylpropanoyl group. A prodrug for zofenoprilat.
Manufacturing Process
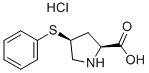
9.9 g (0.031 mole) of cis-4-phenylthio-L-proline is suspended in 100 ml of
water (pH 5.6) and the pH is adjusted to 10.2 by the addition of about 20 ml
of 10% sodium bicarbonate to provide a clear solution. The pH is then
adjusted to 9.5 by the addition of about 4.5 ml of concentrated HCl. The
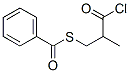
solution is kept at 30°C while 8.1 g (0.033 mole) of (D)-3-(benzoylthio)-2-
methylpropanoic acid chloride in 30 ml of toluene is added simultaneously
with 100 ml of 10% sodium bicarbonate to keep the pH at 9.3. After about
1/4 of the acid chloride is added, a slimy precipitate begins to form which
persists throughout the reaction. After stirring the reaction mixture at pH 9.3
for 2.5 h, it is made strongly acidic by adding 20% HCl in the presence of
ethyl acetate. The aqueous layer is extracted twice with 350 ml portions of
ethyl acetate and the combined organic layers are washed with 300 ml of
saturated brine and dried (MgSO 4 ). The solvent is removed to yield 11.8 g of
foamy solid cis-1-[D-3-(benzoylthyo)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline hydrochloride.
To a solution of this cis-1-[D-3-(benzoylthyo)-2-methyl-1-oxopropyl]-4-
(phenylthio)-L-proline hydrochloride 11.8 g (0.027 mole) in 70 ml of
acetonitrile there is added about 6.0 g of dicyclohexylamine in 25 ml of ether.
A white crystalline precipitate forms immediately. After standing overnight in
the cold room, the solid is filtered and washed with ether to yield (cis)-1-[D-
3-(benzoylthio)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline,
dicyclohexylamine salt (1:1).
he slightly moist (cis)-1-[D-3-(benzoylthio)-2-methyl-1-oxopropyl]-4-
(phenylthio)-L-proline dicyclohexylamine salt is stirred for 2.5 h in a mixture
of 300 ml of ethyl acetate and 200 ml of 10% potassium bisulfate. Two clear
layers form. The aqueous layer is extracted with two 200 ml portions of ethyl
acetate and the combined organic layers are dried (MgSO 4 ). The solvent is
removed to yield 10.1 g of foamy solid (cis)-1-[D-3-(benzoylthio)-2-methyl-1-
oxopropyl]-4-(phenylthio)-L-proline; melting point 42-44°C.
In practice it is usually used as calcium salt (2:1).
brand name
Zoprace (Bristol-Myers Squibb).
Therapeutic Function
Antihypertensive
Synthesis Reference(s)
The Journal of Organic Chemistry, 33, p. 1675, 1968 DOI: 10.1021/jo01268a091
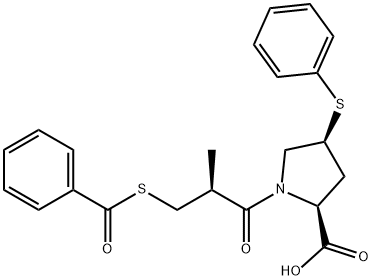
Zofenopril Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| WinWin Chemical CO., Limited | +86-0086-577-64498589 +86-15355981851 | sales@win-winchemical.com | China | 13854 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9358 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29271 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25363 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 20000 | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | Ryan@jiutian-bio.com | China | 7433 | 58 |
Related articles
- General description and determination of 1-Naphthylacetic acid
- 1-Naphthaleneacetic acid (NAA) is naphthalene derivative used as a synthetic plant growth stimulator.
- Jul 15,2022
View Lastest Price from Zofenopril manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-06-26 | Zofenopril
81872-10-8
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Guanlang Biotechnology Co,.LTD | |
 |
2023-04-12 | Zofenopril-d5
81872-10-8
|
US $1.00 / kg | 1kg | 99% | 2000tons/year | Wuhan Dujiang Industrial Co., Ltd. | |
 |
2021-08-11 | Zofenopril-d5 USP/EP/BP
81872-10-8
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited |
-

- Zofenopril
81872-10-8
- US $5.00-2.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD
-

- Zofenopril-d5
81872-10-8
- US $1.00 / kg
- 99%
- Wuhan Dujiang Industrial Co., Ltd.
-

- Zofenopril-d5 USP/EP/BP
81872-10-8
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited





