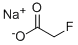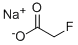SODIUM FLUOROACETATE
- CAS No.
- 62-74-8
- Chemical Name:
- SODIUM FLUOROACETATE
- Synonyms
- smfa;1080;tl869;FRATOL;furatol;Na2AsHO4;compd1080;latka1080;yasoknock;fluorakil3
- CBNumber:
- CB1739103
- Molecular Formula:
- C2H2FNaO2
- Molecular Weight:
- 100.02
- MDL Number:
- MFCD00002682
- MOL File:
- 62-74-8.mol
| Melting point | 200-205 °C (dec.) |
|---|---|
| Boiling point | 105-106 °C |
| vapor pressure | Non-volatile |
| solubility | DMSO (Sparingly), Methanol (Slightly) |
| pka | 2.66 |
| Water Solubility | Very soluble |
| form | Fine white powder |
| Merck | 13,4194 |
| Stability | Stable. Flammable. Risk of explosion above flashpoint. |
| CAS DataBase Reference | 62-74-8(CAS DataBase Reference) |
| EWG's Food Scores | 3-4 |
| FDA UNII | 166WTM3843 |
| Proposition 65 List | Sodium Fluoroacetate |
| Pesticides Freedom of Information Act (FOIA) | Compound 1080 |
| EPA Substance Registry System | Sodium fluoroacetate (62-74-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300-H310-H330-H400 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P262-P264-P270-P280-P302+P350-P310-P322-P361-P363-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P273-P391-P501 |
| Hazard Codes | T+,N,T |
| Risk Statements | 26/27/28-50 |
| Safety Statements | 13-22-36/37-45-61 |
| RIDADR | UN 2629 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS | AH9100000 |
| F | 3 |
| Hazard Note | Highly Toxic |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| HS Code | 29159000 |
| IDLA | 2.5 mg/m3 |
SODIUM FLUOROACETATE Chemical Properties,Uses,Production
Description
Sodium fluoroacetate is the salt of a naturally occurring toxin which is found in Australia, Brazil, and Africa. Naturally occurring fluoroacetate can be found in Gastrolobium minus (family: Fabaceae), a flowering plant in Western Australia and often referred to as the ‘poison pea.’ Descriptions of the fluoroacetate activity may have been described as early at 1904 in Sierra Leone, when colonists used extracts of Chailletia toxicaria to poison rats. The actions of fluoroacetate have also been described in animals having ingested the poison leaf Gifblaar (Dichapetalum cymosum). Sodium fluoroacetate was then developed as a rodenticide and predacide in 1942 in the United States and went under the synonym of 1080, which is its catalog number. In the late 1970s, the use of sodium fluoroacetate was significantly restricted in the United States due to its high acute toxicity and the need for specialized training for application. Additional restrictions were also imposed as to the locations that the agent could be used and under very defined conditions. A ‘toxic collar’ was developed which contains a very small amount of sodium fluoroacetate (0.3 mg per collar). This collar could be placed around the throats of livestock and would contain chemical pouches that would be ruptured when the animal was attacked by a predator thus restricting the poison only to the predator. The toxicity in certain predators (dogs, wolves, coyotes) is up to 20-fold higher than in humans. Amphibians and other reptiles have been shown to be relatively resistant to sodium fluoroacetate and can feed on insects and other animals which have high levels of sodium fluoroacetate present with no ill-effects. In the late 1980s, all use of sodium fluoroacetate as a rodenticide was discontinued. The availability of products containing sodium fluoroacetate is permitted by the Environmental Protection Agency (EPA) and the regulatory conclusion of the EPA is that these products will not pose unreasonable risks or adverse effects if the products are used following the restriction on the product labeling.
Chemical Properties
white odourless hygroscopic powder
Chemical Properties
Sodium fluoroacetate is a fluffy, colorless, odorless, hygroscopic solid (sometimes dyed black).
Uses
Rodenticide (restricted use)
Uses
Sodium fluoroacetate is used for rodent control in restricted environments and for the control of the brushtail possum and other wild mammals in some countries.
Uses
Predator elimination (coyotes), rodenticide.
Definition
ChEBI: Sodium fluoroacetate is an organic sodium salt. It has a role as a rodenticide. It contains a fluoroacetate.
General Description
A fine, white, odorless, powdered solid. Toxic by ingestion, inhalation and skin absorption. Used as a rodenticide.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as SODIUM FLUOROACETATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Toxic by ingestion, inhalation, and skin absorption. For use by trained operators only, use has been restricted. Cardiac impairment, central nervous system impairment, and nausea.
Health Hazard
SODIUM FLUOROACETATE is super toxic. The probable oral lethal dose in humans is less than 5 mg/kg, or a taste (less than 7 drops) for a 150-lb. person.
Fire Hazard
When heated to decomposition, SODIUM FLUOROACETATE emits highly toxic fumes of sodium oxide and fluorides. Avoid decomposing heat.
Agricultural Uses
Insecticide, Rodenticide, Wildlife control: A U.S. EPA restricted Use Pesticide (RUP). Not listed for use in EU countries. It is seldom used as an insecticide. Sodium fluoroacetate is primarily used around sewers, ships and warehouses, and in agriculture and by state agricultural departments as bait for rodents and large predators, to control rats, mice, squirrels, prairie dogs, coyotes, rabbits and otherpests. It is seldom used as an insecticide. It is very toxic to birds, domestic animals and wildlife either by consuming the bait or eating poisoned carcasses. It is sometimes used in 1% solutions which are injected into collars which are strapped to the necks of sheep, goats and other livestock that predators are attracted to. Coyotes that puncture the collars are likely to be fatally poisoned by the sodium fluoroacetate as a result.
Trade name
AI3-08434®; FLUORAKIL® 3; FRATOL®; FURATOL®; RATBANE 1080®; TENEIGHTY®; TL 869®; YASOKNOCK®
Safety Profile
A deadly human poison by ingestion. Experimental poison by ingestion, skin contact, intraperitoneal, subcutaneous, and intravenous routes. A very highly toxic water-soluble salt used mainly as an immediate-action rodenticide. It is absorbed rapidly by the gastrointestinal tract but slowly by the skin unless the skin is abraded or cut. It operates by blocking the Krebs cycle by formation of fluorocitric acid, which inhbits aconitase. It has an effect on either the cardiovascular or nervous system, or both, in all species and, in some species, the skeletal muscles. Humans have mixed responses, with the cardiac feature predominating. By a duect action on the heart, contractile power is lost, which leads to declining blood pressure. Ventricular premature contractions and arrhythmias are seen in all species, including humans. The central nervous system is directly attacked by sodtum fluoroacetate. In humans, the action on the central nervous system produces epileptiform convulsive seizures followed by severe depression. The dangerous dose for humans is 0.5-2 mg/kg. Other species vary considerably in their response to hs material, with primates and birds being the most resistant and carnivora and rodents being the most susceptible. Most domestic animals show a susceptibility fahng between the two extremes indtcated above. Experimental reproductive effects. When heated to decomposition it emits highly toxic fumes of NazO and F-.
Potential Exposure
A potential danger to those involved in the manufacture, formulation, and application of this highly toxic, immediate-action rodenticide.
Carcinogenicity
In developmental studies in rats 0.75mg/kg/day administered by gavage on days 6–17 of gestation caused significant reductions in maternal and fetal body weight gains, but no external fetal abnormalities were noted.
Environmental Fate
Ingestion, inhalation, and dermal exposures are all possible, but ingestion is the major route of exposure. Sodium fluoroacetate is rapidly absorbed by the gastrointestinal tract and by the lungs. There is evidence that leaching and metabolism are the major routes of dissipation. Sodium fluoroacetate which has not undergone degradation is considered mobile by the EPA and has a high risk for movement into the soil and the ground water. Once adsorbed in soil, sodium fluoroacetate can be degraded by halidohydrolase in many microbial and fungal species. The ‘half-life’ of sodium fluoroacetate in soil is dependent on temperature, weather, and initial amount of chemical and decomposition of the host animal. There have been no reports that sodium fluoroacetate can leach into water and reach levels exceeding that which would be deemed toxic.
Metabolic pathway
Sodium fluoroacetate is toxic to all mammals, especially to dogs (LD50
0.1 mg kg-1) and therefore its use is very restricted. An exception is the
aerial application of baits for the control of the brushtail possum in
New Zealand and of other wild mammals in Australia. Such use is controversial
and has been the impetus for some environmental fate studies
(Eason et al., 1993) and secondary toxicity studies.
The role of fluoroacetate in interfering with citrate metabolism is now
classical biochemical toxicology (see below).
Shipping
UN2629 Sodium fluorosilicate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
It is a free-flowing white HIGHLY TOXIC powder which is purified by dissolving it in ca 4 parts of H2O and the pH is checked. If it is alkaline, add a few drops of FCH2CO2H to make the solution just acidic. Evaporate (fumehood) on a steam bath until crystals start to separate, cool and filter the solid off. More solid can be obtained by adding EtOH to the filtrate. Dry it at 100o in vacuum. The p-nitrobenzyl ester crystallises from EtOH with m 76o. POISONOUS. The free acid interferes with the citric acid cycle. [Saunders & Stacey J Chem Soc 1778 1948, Beilstein 2 IV 446.]
Degradation
Sodium fluoroacetate is a stable hygroscopic crystalline solid which is very soluble in water. In the absence of biodegradation it is stable in water.
Toxicity evaluation
Fluoroacetate produces its toxic action (after conversion to fluorocitrate) by inhibiting the Krebs cycle. The compound is incorporated into fluoroacetyl coenzyme A, which condenses with oxaloacetate to form fluorocitrate. This inhibits the enzyme aconitase, which inhibits conversion of citrate to cis-aconitic acid/ isocitrate. This inhibition will lead to a buildup of citric acid resulting in convulsions and death from cardiac failure or respiratory arrest. Mitochondrial uptake of acetate may also be affected. The heart and CNS are the tissues most affected by this inhibition of oxidative energymetabolism.Oxygen consumption is markedly reduced. In addition to blockade of energy production, depletion of calcium may also be involved in the clinical manifestations associated with sodium fluoroacetate toxicity.
Incompatibilities
Incompatible with alkaline metals and carbon disulfide. Avoid decomposing heat.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. This compound is unstable at temperatures above 110°C and decomposes @ 200°C. Thus, careful incineration has been suggested as a disposal procedure. According to their procedure, the produce should be mixed with large amounts of vermiculite, sodium bicarbonate, and sand-soda ash. Slaked lime should also be added to the mixture. Two incineration procedures for this mixture are suggested. The better of these procedures is to burn the mixture in a closed incinerator equipped with an afterburner and an alkali scrubber. The other procedure suggests that the mixture be covered with scrap wood and paper in an open incinerator. (The incinerator should be lighted by means of an excelsior train).
SODIUM FLUOROACETATE Preparation Products And Raw materials
Raw materials
Preparation Products
62-74-8(SODIUM FLUOROACETATE)Related Search:
1of4