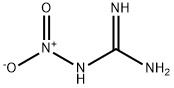
Nitroguanidine
- CAS No.
- 556-88-7
- Chemical Name:
- Nitroguanidine
- Synonyms
- 2-Nitroguanidine;Picrite;NIGU;NSC 41036;NITROGUANIDINE;nitro-guanidin;Nitro uanidine;1-nitro-guanidin;1-Nitroguanidine;Guanidine,nitro-
- CBNumber:
- CB1853823
- Molecular Formula:
- CH4N4O2
- Molecular Weight:
- 104.07
- MDL Number:
- MFCD00007039
- MOL File:
- 556-88-7.mol
- MSDS File:
- SDS
| Melting point | 239 °C (dec.) (lit.) |
|---|---|
| Boiling point | 195.15°C (rough estimate) |
| Density | 1.55g/cm3 |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.6850 (estimate) |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 3.45±0.50(Predicted) |
| color | White to Off-White |
| Water Solubility | 3 g/L (20 ºC) |
| Merck | 14,6607 |
| BRN | 1756640 |
| LogP | -0.815 at 20℃ |
| CAS DataBase Reference | 556-88-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | NAY6KWL67F |
| NIST Chemistry Reference | Guanidine, nitro-(556-88-7) |
| EPA Substance Registry System | Nitroguanidine (556-88-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H208 | |||||||||
| Precautionary statements | P210-P212-P230-P233-P280-P371+P380+P375-P501 | |||||||||
| Hazard Codes | F,Xi | |||||||||
| Risk Statements | 11-36/37/38-5 | |||||||||
| Safety Statements | 16-26-33-36/37/39-47-37/39 | |||||||||
| RIDADR | UN 1336 4.1/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | MF4600000 | |||||||||
| HazardClass | 1.1D | |||||||||
| PackingGroup | I | |||||||||
| NFPA 704 |
|
Nitroguanidine price More Price(9)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | N17351 | Nitroguanidine | 556-88-7 | 100g | $40 | 2024-03-01 | Buy |
| Sigma-Aldrich | N17351 | Nitroguanidine | 556-88-7 | 500g | $146 | 2024-03-01 | Buy |
| Usbiological | 460807 | Nitroguanidine | 556-88-7 | 100mg | $319 | 2021-12-16 | Buy |
| TRC | N496000 | Nitroguanidine(Wettedwithupto20%water) | 556-88-7 | 500mg | $120 | 2021-12-16 | Buy |
| AK Scientific | 5286EA | Nitroguanidine | 556-88-7 | 25g | $90 | 2021-12-16 | Buy |
Nitroguanidine Chemical Properties,Uses,Production
Chemical Properties
Nitroguanidine is a white powder, It is a well known explosive substance which is used both in high ex plosives and in propellent powders. As a high explosive it is about as powerful as trinitrotoluene and has been used in various mixtures, with ammonium nitrate, paraffin, etc., in bombs and for other purposes where an insensitivir and powerful high explosive is needed. It has the further advantage of be ing exceptionally cool. The experiments of Vieille showed that this explosive produces a temperature of only 907 C. Its coolness commends it for use in safety explosives. If incorporated into nitrocellulose powder it renders the powder flashless without impair ing its ballistic power, and, as Vieille showed, reduces erosion and lengthens the accuracy life of the gun.
Physical properties
Nitroguanidine [556-88-7], CH4N4O2, Mr 104.07, is a very weak base that forms salts only with strong acids. Its solubility in water at 20 ℃ (100 ℃) is 0.27 % (7.6 %). It decomposes at 220 ℃.
Uses
Nitroguanidine is used in the prediction of heats of detonation of energetic compounds from their molecular structure. It is also used in the synthetic preparation of imidacloprid-d4 (I274992), which is a neonicotinoid.
Reactant involved in the synthesis of:
1,5-Disubstituted-1,3,5-hexahydrotriazine-2-(N-nitro)imines with insecticidal activity
Hexahydrotriazine-N-nitroimine analogs with insecticidal activity via Mannich reactions
Reactant involved in:
The study of the effects of vessel materials on exothermic decomposition energy
Nitration of arenes
Nitration of (nitrimino)tetrahyrdooxadiazine and (nitrimino)hexahydrotriazine
Uses
Reactant involved in the synthesis of:
- 1,5-Disubstituted-1,3,5-hexahydrotriazine-2-(N-nitro)imines with insecticidal activity
- Hexahydrotriazine-N-nitroimine analogs with insecticidal activity via Mannich reactions
Reactant involved in:
- The study of the effects of vessel materials on exothermic decomposition energy
- Nitration of arenes
- Nitration of (nitrimino)tetrahyrdooxadiazine and (nitrimino)hexahydrotriazine
Definition
ChEBI: 1-nitroguanidine is a nitroguanidine and a one-carbon compound. It is a tautomer of a 2-nitroguanidine.
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 2327, 1951 DOI: 10.1021/ja01149a124
Organic Syntheses, Coll. Vol. 1, p. 399, 1941
General Description
Nitroguanidine is shipped as a slurry or wet mass of pale yellow crystals. If Nitroguanidine should dry out Nitroguanidine can explode due to shock, heat, flame, or friction. The primary hazard is blast where the entire load can explode instantaneously and not from flying projectiles and fragments. Under prolonged exposure to fire or heat they can explode.
Reactivity Profile
Nitroalkanes, such as NITROGUANIDINE, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive. Nitroalkanes are insoluble in water. Flammable/combustible material. May be ignited by heat, sparks or flames. DRIED OUT material may explode if exposed to heat, flame, friction or shock; Treat as an explosive. Mercury and silver complex salts of nitroguanidine are very impact sensitive.
Hazard
May explode when shocked or heated.
Health Hazard
Some are toxic and may be fatal if inhaled, swallowed or absorbed through skin. Contact may cause burns to skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Health Hazard
Toxicity of nitroguanidine is very low in test animals. It is nontoxic in rats when given by oral administration at doses as high as 1000 mg/kg/day for 14 days (Morgan et al.1988). The LD50 value, as determined by the oral gavage single-dose limit test, is >5000 mg/kg in both male and female rats (Brown et al. 1988).
Fire Hazard
Nitroguanidine is an explosive of moderate power. Its brisance is less than that of TNT or trinitrobenzene. It requires an initiator to explode. On the other hand, nitroguanidine acts as a sensitizer, causing many nonexploding fuel-air systems detonable. Tulis (1984) investigated the detonation of unconfined clouds of aluminum powder in air sensitized with a small amount of nitroguanidine. Aluminum powder dispersed in air becomes more readily detonable when mixed with nitroguanidine. The latter added to a mixture of aluminum powder and potassium chlorate causes transition of the deflagrating mixture into detonable (Tulis et al. 1984). A similarly insensitive explosive, ammonium chlorate, becomes readily detonable when combined with nitroguanidine or nitroguanidine and aluminum powder. Aluminum powder added to a detonable composition of nitroguanidine, and NH4ClO4, caused degradation of the detonation velocity. Moist nitroguanidine containing about 25% water is a flammable solid.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A very dangerous fire hazard when exposed to heat, flame, or by chemical reaction with oxidizers. A severe explosion hazard when shocked or exposed to heat or flame. It is about as powerful as TNT. It is normally mixed with colloided nitrocellulose or ammonium nitrate and paraffin wax. Can react vigorously with oxidizing materials and the derivatives can be explosive. The mercury and silver salts and other derivatives are much more impact-sensitive. When heated to decomposition it emits highly toxic fumes of NOx. See also NITRO COMPOUNDS.
Synthesis
Nitroguanidine is made by treating guanidine nitrate with concentrated sulfuric acid, fuming nitric acid, or both. The reaction of nitroguanidine with amines gives substituted nitroguanidines.
Purification Methods
Crystallise it from water (20mL/g). The nitrate has m 147o(dec)( prisms, H2O). [Beilstein 3 H 126, 3 III 236.]
Nitroguanidine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| CD Chemical Group Limited | +8615986615575 | info@codchem.com | China | 20356 | 58 |
View Lastest Price from Nitroguanidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-02-04 | Nitroguanidine
556-88-7
|
US $0.00-0.00 / KG | 1KG | 99% | 20 mt | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-01-31 | Nitroguanidine
556-88-7
|
US $50.00 / KG | 1KG | 99% | 5000KG | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-10-22 | Nitroguanidine
556-88-7
|
US $9.90 / kg | 1kg | 99% | 200MT/mouth | Hebei Duling International Trade Co. LTD |
-

- Nitroguanidine
556-88-7
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Nitroguanidine
556-88-7
- US $50.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Nitroguanidine
556-88-7
- US $9.90 / kg
- 99%
- Hebei Duling International Trade Co. LTD