o-Xylene
- CAS No.
- 95-47-6
- Chemical Name:
- o-Xylene
- Synonyms
- XYLENES;1,2-DIMETHYLBENZENE;XYLOL;1,2-Xylene;o-Xylol;YLENE;ORTHO-XYLENE;2-methyltoluene;o-Dimethylbenzene;2-xylene
- CBNumber:
- CB1854693
- Molecular Formula:
- C8H10
- Molecular Weight:
- 106.17
- MDL Number:
- MFCD00008519
- MOL File:
- 95-47-6.mol
- MSDS File:
- SDS
| Melting point | -26--23 °C (lit.) |
|---|---|
| Boiling point | 143-145 °C (lit.) |
| Density | 0.879 g/mL at 20 °C (lit.) |
| vapor density | 3.7 (vs air) |
| vapor pressure | <0.1 atm ( 21.1 °C) |
| refractive index |
n |
| Flash point | 90 °F |
| storage temp. | Store below +30°C. |
| solubility | water: partially soluble0.1705 g/L at 25°C |
| form | Solid or Crystalline Powder |
| pka | >15 (Christensen et al., 1975) |
| color | Yellow to beige |
| Odor | Benzene-like; characteristic aromatic. |
| explosive limit | 1.0-7.6%(V) |
| Odor Threshold | 0.38ppm |
| Viscosity | 0.75mm2/s |
| Water Solubility | Sparingly soluble in water. (0.2g/L) |
| λmax |
λ: 288 nm Amax: 1.00 λ: 300 nm Amax: 0.40 λ: 325 nm Amax: 0.05 λ: 350-400 nm Amax: 0.01 |
| Merck | 14,10081 |
| BRN | 1815558 |
| Henry's Law Constant | 14.0 at 45.00 °C, 16.4 at 50.00 °C, 19.0 at 55.00 °C, 21.3 at 60.00 °C, 26.3 at 70.00 °C (static headspace-GC, Park et al., 2004) |
| Exposure limits | NIOSH REL: 100 ppm (435 mg/m3), STEL 150 ppm (655 mg/m3), IDLH 900 ppm; OSHA PEL: TWA 100 ppm; ACGIH TLV: TWA 100 ppm, STEL 150 ppm (adopted). |
| Dielectric constant | 2.5699999999999998 |
| Stability | Stable. Incompatible with oxidizing agents. Flammable. Hygroscopic. |
| InChIKey | CTQNGGLPUBDAKN-UHFFFAOYSA-N |
| EPA Primary Drinking Water Standard | MCL:10,MCLG:10 |
| LogP | 3.16 at 20℃ |
| CAS DataBase Reference | 95-47-6(CAS DataBase Reference) |
| EWG's Food Scores | 7 |
| FDA UNII | Z2474E14QP |
| NIST Chemistry Reference | Benzene, 1,2-dimethyl-(95-47-6) |
| EPA Substance Registry System | o-Xylene (95-47-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H304-H312+H332-H315-H335-H412 | |||||||||
| Precautionary statements | P210-P273-P280-P301+P310-P303+P361+P353-P331 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 10-20/21-38-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 25-45-36/37-16-7 | |||||||||
| RIDADR | UN 1307 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | ZE2450000 | |||||||||
| Autoignition Temperature | 867 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29024100 | |||||||||
| Toxicity | Acute oral LD50 in rats is approximately 5 g/kg (quoted, RTECS, 1992). | |||||||||
| IDLA | 900 ppm | |||||||||
| NFPA 704 |
|
o-Xylene price More Price(53)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | XX0020 | o-Xylene Reagent | 95-47-6 | 4L | $364 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.08697 | o-Xylene for synthesis | 95-47-6 | 1L | $123 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.08697 | o-Xylene for synthesis | 95-47-6 | 2.5L | $233 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.08697 | o-Xylene for synthesis | 95-47-6 | 10L | $552 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40201 | o-Xylene solution certified reference material, 5000?μg/mL in methanol | 95-47-6 | 1mL | $63.8 | 2024-03-01 | Buy |
o-Xylene Chemical Properties,Uses,Production
Xylene
Xylene refers to the aromatic hydrocarbon with the two hydrogen atoms on the benzene ring being substituted by two methyl groups. It has three isomers o-xylene (1, 2-Dimethylbenzene), m-xylene and p-xylene. The industrial products are the mixtures of the three isomers with 10% o-10%, 70% m-, and 20% p-. In the coking industry, it is one of crude benzene refined products.
Xylene is a kind of colorless flammable liquid; the melting point of o-, m-and p-xylene is-25.2 ℃,-47.9 ℃ and 13.3 ℃; the boiling points are respectively 144.4 ℃, 139.1 ℃ and 138.3 ℃ while the relative density is 0.8802, 0.8642 and 0.8611, respectively; It is not soluble in water but miscible with many kinds of organic solvents immiscibility. Upon catalytic oxidation, they respectively, generate phthalic anhydride, isophthalic acid and terephthalic acid.
Xylene is one kind of important raw materials of organic chemicals, naturally existing in coal tar and some kinds of petroleum. It can be obtained through the fractionation of the light oil part of the coal tar or catalytic reforming light gasoline. Industry mainly performs extracting using the C8 fraction in the naphtha reformates. It can be alternatively manufactured through the disproportionation reaction of toluene in the presence of catalyst and high temperature, high pressure. At present time, industry mainly applies the method of cryogenic crystallization, adsorption and formation of complexes or molecular sieves to separate them. O-xylene has a relatively high boiling point, being able to be separated using distillation. p-xylene also has a high melting point and can be purified through fractional crystallization purification. Mixed xylene without separation can be directly used as a solvent with being supplemented to the gasoline capable of improving the anti-explosive properties. They are components of aviation gasoline. O-xylene is mainly used for the preparation of phthalic anhydride, which is an important raw material for the manufacture of a variety of dyes and indicators (such as phenolphthalein). In addition, o-xylene can also be used for preparation of polyester resin, insect repellent, plasticizers and dyes. M-xylene, through nitration and reduction, can generate 4, 6-dimethyl-1, 3-phenylenediamine that is the intermediate for synthetic dyes. M-xylene can also be used as the raw materials for synthetic fragrances (such as xylene musk). P-xylene is mainly used in the manufacture of terephthalic acid, which is an important raw material for synthetic polyester fiber (polyester).
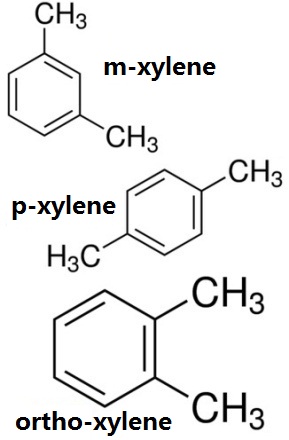
Figure 1 the chemical structure of the three isomers of xylene, ortho-xylene, m-xylene, p-xylene chemical structure.
The above information is edited by Chemicalbook.
Precision Distillation for separation of O-xylene and p-xylene
Xylene is presented in coked crude benzene and petroleum cracked oil. Crude benzene, after initial distillation, sulfuric acid washing and distillation for separation of benzene and toluene, followed by distillation, we can obtain xylene, also known as coking xylene. The quality of the coked xylene depends on the separation capacity of the distillation column, the temperature at the top of the column and the reflux ratio. China has classified the coking xylene products into three levels. The coking xylene generally contains 16% if o-xylene, 50% of m-xylene, 21% of p-xylene and 7% of ethylbenzene. The xylene produced in the petroleum industry has a low content of m-xylene and a high content of ethylbenzene. Industrial xylene is not only the solvent and additive of rubber and coatings industry, but also the additives of aviation and power fuel. O-xylene, m-xylene and p-xylene separated from industrial xylene are the raw materials of phthalic acid, isophthalic acid and terephthalic acid, respectively. Phthalic acid and terephthalic acid are used in the production of plasticizers, polyester resins and polyester fibers. M-xylene can be used alone as solvent and fuel additives. The o-xylene contained in the industrial xylene has a over 5.2 ℃ difference with other isomers. With precision distillation, we can obtain o-xylene with a purity of over 95%, followed by using sulfonation and distillation for purification so we can get further purer o-xylene.
Xylene belongs to Lewis base, which can form a polar complex with HF-BF3 (Lewis acid). The alkalinity of M-xylene is about 100 times as strong as that of other C8 aromatics. When the isomer mixture of xylene comes into contact with HF-BF3 solvent, m-xylene can form a complex with fluoride and is preferentially extracted into the fluoride phase. The m-xylene-containing fluoride phase is heated at a lower pressure to decompose the complex, thereby separating m-xylene from the mixture. HF-BF3 solvent can be recovered by distillation for recycling. If the raw material is a mixture of ortho-xylene, m-xylene and p-xylene, after the m-xylene is extracted, we can further use precision distillation to separate the o-xylene and p-xylene.
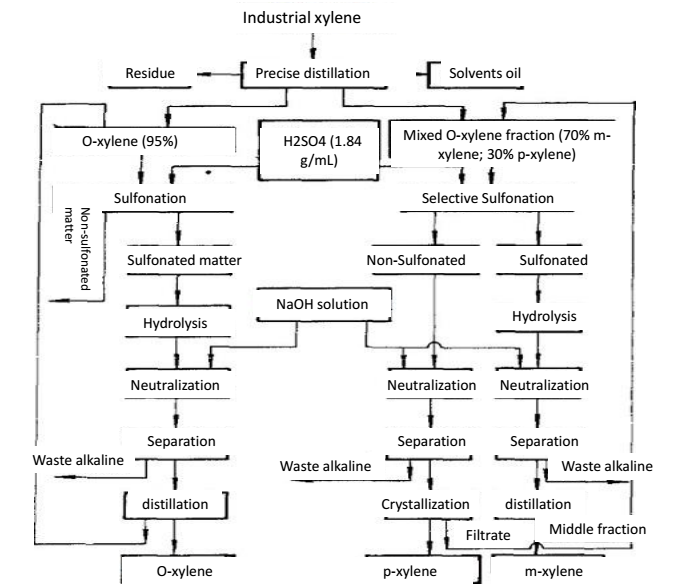
Figure 2 the precision distillation method for separation of o-xylene and p-xylene.
Chemical properties
It appears as colorless transparent liquid with aromatic odor. It is miscible with ethanol, ethyl ether, acetone and benzene but insoluble in water.
Uses
(1) It is mainly used in the production of phthalic anhydride
(2) O-xylene is the raw material for the production of germicide fenramine, tetrachlorophenyl peptide and the herbicide bensulfuron-methyl. It is used as intermediate for the manufacture of o-methyl benzoic acid.
(3) It is mainly used as chemical raw materials and solvents. It can be used to produce phthalic anhydride, dyes, pesticides and drugs, such as vitamins. It can also be used as aviation gasoline additives.
(4) Used as chromatographic standards and solvents
(5) As raw materials of synthesis of anhydride and other organic synthesis;
Production method
Industry applied super-distillation method to separate out the o-xylene from the mixed xylene. O-xylene has a over 5 ℃ difference in the boiling point compared with other components in the mixed xylene. For the distillation, the required tray number is about 150; the reflux ratio being 5-8 and consume relative much energy.
O-xylene was originally produced mainly from coal tar. Currently most of the domestic and foreign production of o-xylene is mainly via extraction from oil catalytic reforming and thermal cracking of aromatic hydrocarbon. Owing to that the structures of o-xylene, p-xylene, and m-xylene in the xylene are very similar; their physical parameters are also quite similar. Industrial o-xylene separation mainly adopts super-distillation method; first separate out the o-xylene and ethylbenzene from the mixed xylene which demands the using of 100~150 tray distillation tower; followed by separation of o-xylene and ethylbenzene to obtain pure o-xylene.
Category
Flammable liquids
Toxicity grading
poisoning
Acute toxicity
Oral-rat LDL0: 5000 mg/kg; abdominal injection-mouse LD50: 1364 mg/kg
EXPLOSIVES and HAZARDOUS CHARACTERISTICS
being explosive when mixed with air
Flammability and Hazardous characteristics
being flammable upon flame, heat, oxidant Flammable with combustion releasing irritant smoke
Storage and transportation characteristics
warehouse: ventilated, low temperature and dry; gently load and unload; store it separately from oxidants and acids.
Fire extinguishing agent
mist water, foam, sand, carbon dioxide, 1211 extinguishing agent
Occupational Standard
TLV-TWA 100 PPM (440 mg/m 3); STEL 150; PPM (655 mg/m 3)
Chemical Properties
colourless liquid
Physical properties
Clear, colorless liquid with an aromatic odor. An odor threshold concentration of 380 ppbv was reported by Nagata and Takeuchi (1990).
Uses
Preparation of phthalic acid, phthalic anhydride, terephthalic acid, isophthalic acid; solvent for alkyd resins, lacquers, enamels, rubber cements; manufacture of dyes, pharmaceuticals, and insecticides; motor fuels.
Uses
o-Xylene is largely used in the production of phthalic anhydride, and is generally extracted by distillation from a mixed Xylene stream in a plant primarily designed for p-Xylene production.
Uses
Xylene, xylenes, and total xylenes are used interchangeably since xylene usually exists as a mixture of three isomers: 1,2-dimethylbenzene, 1,3-dimethylbenzene, and 1,4-dimethylbenzene, i.e., o-, m-, and p-xylene, and is usually used as a mixture of the three forms. The mixture often also contains ethylbenzene. It is a high volume industrial chemical used in the synthetic fiber, chemical and plastics industries and as a solvent, cleaning agent and thinner for paints and varnishes.
Definition
ChEBI: A xylene substituted by methyl groups at positions 1 and 3.
Synthesis Reference(s)
Journal of the American Chemical Society, 97, p. 7262, 1975 DOI: 10.1021/ja00858a011
The Journal of Organic Chemistry, 44, p. 2185, 1979 DOI: 10.1021/jo01327a032
General Description
A colorless watery liquid with a sweet odor. Less dense than water. Insoluble in water. Irritating vapor.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
1,2-Dimethylbenzene may react with oxidizing materials. .
Health Hazard
Vapors cause headache and dizziness. Liquid irritates eyes and skin. If taken into lungs, causes severe coughing, distress, and rapidly developing pulmonary edema. If ingested, causes nausea, vomiting, cramps, headache, and coma. Can be fatal. Kidney and liver damage can occur.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel considerable distance to a source of ignition and flash back.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic bj7 intraperitoneal route. Mldly toxic by ingestion and inhalation. An experimental teratogen. A common air contaminant. A very dangerous fire hazard when exposed to heat or flame. Explosive in the form of vapor when exposed to heat or flame. To fight fire, use foam, CO2, dry chemical. Incompatible with oxidzing materials. When heated to decomposition it emits acrid smoke and irritating fumes. Emitted from modern building materials (CENEAR 69,22,91). See also other xylene entries.
Source
Detected in distilled water-soluble fractions of 87 octane gasoline (3.83 mg/L), 94 octane
gasoline (11.4 mg/L), Gasohol (8.49 mg/L), No. 2 fuel oil (1.73 mg/L), jet fuel A (0.87 mg/L),
diesel fuel (1.75 mg/L), military jet fuel JP-4 (1.99 mg/L) (Potter, 1996), new motor oil (16.2 to 17.5 μg/L), and used motor oil (294 to 308 μg/L) (Chen et al., 1994). The average volume percent
and estimated mole fraction in American Petroleum Institute PS-6 gasoline are 2.088 and 0.01959,
respectively (Poulsen et al., 1992). Schauer et al. (1999) reported o-xylene in a diesel-powered
medium-duty truck exhaust at an emission rate of 830 μg/km. Diesel fuel obtained from a service
station in Schlieren, Switzerland contained o-xylene at a concentration of 223 mg/L (Schluep et
al., 2001).
California Phase II reformulated gasoline contained o-xylene at a concentration of 19.7 g/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 5.41 and 562 mg/km, respectively (Schauer et al., 2002).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
602. Average m+p-xylene concentrations reported in water-soluble fractions of unleaded gasoline,
kerosene, and diesel fuel were 8.611, 0.658, and 0.228 mg/L, respectively. When the authors
analyzed the aqueous-phase via U.S. EPA approved test method 610, average m+p-xylene
concentrations in water-soluble fractions of unleaded gasoline, kerosene, and diesel fuel were
lower, i.e., 6.068, 0.360, and 0.222 mg/L, respectively.
Based on laboratory analysis of 7 coal tar samples, o-xylene concentrations ranged from 2 to
2,000 ppm (EPRI, 1990). A high-temperature coal tar contained o-xylene at an average
concentration of 0.04 wt % (McNeil, 1983).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of o-xylene was 18.1 mg/kg of pine burned. Emission rates of o-xylene were not measured
during the combustion of oak and eucalyptus.
Drinking water standard (final): For all xylenes, the MCLG and MCL are both 10 mg/L. In
addition, a DWEL of 70 mg/L was recommended (U.S. EPA, 2000).
Environmental Fate
Biological. Reported biodegradation products of the commercial product containing xylene
include α-hydroxy-p-toluic acid, p-methylbenzyl alcohol, benzyl alcohol, 4-methylcatechol, mand
p-toluic acids (Fishbein, 1985). o-Xylene was also cometabolized resulting in the formation of
o-toluic acid (Pitter and Chudoba, 1990). In anoxic groundwater near Bemidji, MI, o-xylene
anaerobically biodegraded to the intermediate o-toluic acid (Cozzarelli et al., 1990). In gasolinecontaminated
groundwater, methylbenzylsuccinic acid was identified as the first intermediate
during the anaerobic degradation of xylenes (Reusser and Field, 2002).
Photolytic. Cox et al. (1980) reported a rate constant of 1.33 x 10-11 cm3/molecule?sec for the
reaction of gaseous o-xylene with OH radicals based on a value of 8 x 10-12 cm3/molecule?sec for
the reaction of ethylene with OH radicals.
Surface Water. The evaporation half-life of o-xylene in surface water (1 m depth) at 25 °C is
estimated to be 5.18 h (Mackay and Leinonen, 1975).
Groundwater. Nielsen et al. (1996) studied the degradation of o-xylene in a shallow,
glaciofluvial, unconfined sandy aquifer in Jutland, Denmark. As part of the in situ microcosm study, a cylinder that was open at the bottom and screened at the top was installed through a cased
borehole approximately 5 m below grade. Five liters of water was aerated with atmospheric air to
ensure aerobic conditions were maintained. Groundwater was analyzed weekly for approximately
3 months to determine o-xylene concentrations with time. The experimentally determined firstorder
biodegradation rate constant and corresponding half-life following a 7-d lag phase were
0.1/d and 6.93 d, respectively.
Photolytic. When synthetic air containing gaseous nitrous acid and o-xylene was exposed to
artificial sunlight (λ = 300–450 nm) biacetyl, peroxyacetal nitrate, and methyl nitrate formed as
products (Cox et al., 1980). A n-hexane solution containing o-xylene and spread as a thin film (4
mm) on cold water (10 °C) was irradiated by a mercury medium pressure lamp. In 3 h, 13.6% of
the o-xylene photooxidized into o-methylbenzaldehyde, o-benzyl alcohol, o-benzoic acid, and omethylacetophenone
(Moza and Feicht, 1989). Irradiation of o-xylene at ≈ 2537 ? at 35 °C and 6
mmHg isomerizes to m-xylene (Calvert and Pitts, 1966). Glyoxal, methylglyoxal, and biacetyl
were produced from the photooxidation of o-xylene by OH radicals in air at 25 °C (Tuazon et al.,
1986a).
Chemical/Physical. Under atmospheric conditions, the gas-phase reaction of o-xylene with OH
radicals and nitrogen oxides resulted in the formation of o-tolualdehyde, o-methylbenzyl nitrate,
nitro-o-xylenes, 2,3-and 3,4-dimethylphenol (Atkinson, 1990). Kanno et al. (1982) studied the
aqueous reaction of o-xylene and other aromatic hydrocarbons (benzene, toluene, m- and p-xylene,
and naphthalene) with hypochlorous acid in the presence of ammonium ion. They reported that the
aromatic ring was not chlorinated as expected but was cleaved by chloramine forming cyanogen
chloride. The amount of cyanogen chloride formed increased at lower pHs (Kanno et al., 1982). In
the gas phase, o-xylene reacted with nitrate radicals in purified air forming the following products:
5-nitro-2-methyltoluene and 6-nitro-2-methyltoluene, o-methylbenzaldehyde, and an aryl nitrate
(Chiodini et al., 1993).
Purification Methods
o-Xylene (4.4Kg) is sulfonated by stirring for 4hours with 2.5L of conc H2SO4 at 95o. After cooling, and separating the unsulfonated material, the product is diluted with 3L of water and neutralised with 40% NaOH. On cooling, sodium o-xylene sulfonate separates and is recrystallised from half its weight of water. [A further crop of crystals is obtained by concentrating the mother liquor to one-third of its volume.] The salt is dissolved in the minimum amount of cold water, then mixed with the same amount of cold water, and with the same volume of conc H2SO4 and heated to 110o. o-Xylene is regenerated and steam distils. The distillate is saturated with NaCl, the organic layer is separated, dried and redistilled. [Beilstein 5 H 362, 5 I 179, 5 II 281, 5 III 807, 5 IV 917.]
o-Xylene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7378 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9352 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| NINGBO INNO PHARMCHEM CO., LTD. | 13867897135 | sales@nbinno.com | CHINA | 925 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
Related articles
- o-Xylene: Occurence, Uses, Environmental Fate, Toxicity
- o-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2. with two methyl substituents bonded to adjacen....
- Nov 13,2019
View Lastest Price from o-Xylene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-24 | o-Xylene
95-47-6
|
US $100.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-07-27 | o-Xylene
95-47-6
|
US $1.50 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd | |
 |
2023-06-03 | o-Xylene
95-47-6
|
US $9.00 / KG | 1KG | 99.9 | 1 ton | Hebei Guanlang Biotechnology Co,.LTD |
95-47-6(o-Xylene)Related Search:
1of4