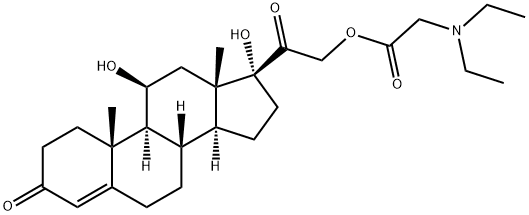
hydrocortamate
- CAS No.
- 76-47-1
- Chemical Name:
- hydrocortamate
- Synonyms
- hydrocortamate;FWFVLWGEFDIZMJ-FOMYWIRZSA-N;Glycine, N,N-diethyl-, (11β)-11,17-dihydroxy-3,20-dioxopregn-4-en-21-yl ester;[2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] 2-(diethylamino)acetate;[2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxo-ethyl] 2-(diethylamino)ethanoate;2-(diethylamino)acetic acid [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-3-keto-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-keto-ethyl] ester
- CBNumber:
- CB1903968
- Molecular Formula:
- C27H41NO6
- Molecular Weight:
- 475.62
- MDL Number:
- MFCD00864163
- MOL File:
- 76-47-1.mol
| Melting point | 162-163° |
|---|---|
| Boiling point | 627.2±55.0 °C(Predicted) |
| Density | 1.21±0.1 g/cm3(Predicted) |
| pka | 12.32±0.70(Predicted) |
| FDA UNII | Y3N00BK5WK |
hydrocortamate Chemical Properties,Uses,Production
Originator
Magnacort,Pfizer,US,1956
Uses
Hydrocortamate is a synthetic glucocorticoid with anti-inflammatory and immunosuppressive properties. It is used topically to treat inflammation due to corticosteroid-responsive dermatoses.
Definition
ChEBI: Hydrocortamate is a glycinyl ester, an 11beta-hydroxy steroid, a 17alpha-hydroxy steroid, a glucocorticoid, a 3-oxo-Delta(4) steroid and a tertiary alpha-hydroxy ketone. It has a role as an anti-inflammatory drug and an immunosuppressive agent. It is functionally related to a cortisone.
Manufacturing Process
1 g of hydrocortisone is introduced with stirring into 5 cc of anhydrous
pyridine. After heating to 45°C and then cooling again to 0°C to 5°C there is
slowly added dropwise a freshly prepared solution of 0.52 g (1 mol + 10%) of
chloracetic anhydride in 4 cc of absolute ether, The reaction temperature
should not exceed 10°C. During the whole time of reaction a stream of
nitrogen is passed through the reaction mixture in order to achieve an
exhaustive evaporation of the added ether. The batch is slowly allowed to
come to room temperature, an operation requiring 4 to 5 hours, and then 0.1
cc of water is added for decomposition of the excess of anhydride. The
reaction solution is introduced dropwise with stirrinq within 1 hour into 100 cc
of water as a result of which the 21-chloracetate of hydrocortisone is
deposited. After filtration with suction, washing is carried out with water, 5%
hydrochloric acid, water, 2% sodium bicarbonate solution and water again.
The substance is then dried in a vacuum desiccator. The white chloracetate
thus obtained melts at 213°C to 214°C with decomposition. It is free from
nitrogen and the yield amounts to 93.4% of the theoretical.
1 g of hydrocortisone-21-chloracetate is dissolved in 15 cc of anhydrous and
peroxide-free tetrahydrofuran. The solution produced is treated with a solution of 0.42 g of diethylamine in 15 cc of tetrahydrofuran. The reaction mixture is
allowed to stand for 24 hours at room temperature. The separated
diethylamine hydrochloride is filtered with suction and the filtrate evaporated
under vacuum in a nitrogen atmosphere at 40°C. The residue is triturated
with a little absolute ether and suction filtered. It is washed on the filter with
a little ether and then with hexane. The 21-diethylaminoacetate of
hydrocortisone melts at 150°C to 162°C. The base can be recrystallized from
ethyl acetate but its melting point remains practically unchanged at 162°C to
163°C. The yield amounts to 72.5% of the theoretical. For conversion of the
base into the hydrochloride it is suspended in ether and the suspension
treated with ethereal hydrochloric acid. The hydrochloride is filtered with
suction and recrystallized from ethanol; MP 222°C with decomposition.
With a starting quantity of 14g, the yield amounted to 85.4% of the
theoretical.
brand name
Magnacort (Pfizer).
Therapeutic Function
Corticosteroid
hydrocortamate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9448 | 58 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing@energy-chemical.com | China | 44941 | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 15229059051 | 1027@dideu.com | China | 9926 | 58 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |
| Shaanxi Didu New Materials Co. Ltd | 58 |
| Energy Chemical | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 58 |