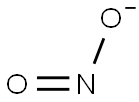
Nitrite
- CAS No.
- 14797-65-0
- Chemical Name:
- Nitrite
- Synonyms
- Nitrous acid, ion(1-);Oxylatonitrogen oxide
- CBNumber:
- CB21236149
- Molecular Formula:
- HNO2
Lewis structure
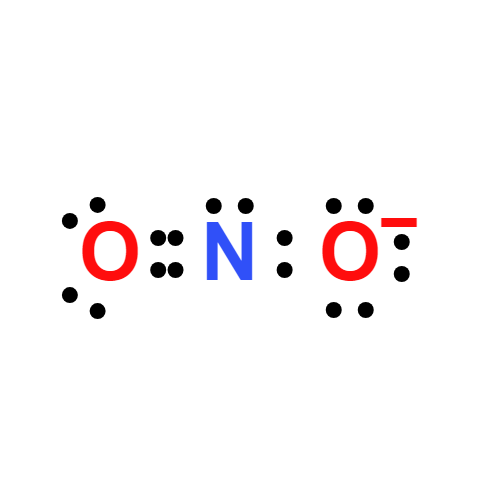
- Molecular Weight:
- 46.01
- MDL Number:
- MFCD00144923
- MOL File:
- 14797-65-0.mol
| EWG's Food Scores | 1-4 |
|---|---|
| FDA UNII | J39976L608 |
| EPA Substance Registry System | Nitrite (14797-65-0) |
Nitrite Chemical Properties,Uses,Production
Definition
ChEBI: The nitrogen oxoanion formed by loss of a proton from nitrous acid.
General Description
Colorless solutions or crystalline solids. Denser than water. Contact may cause irritation to skin, eyes, and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
Reactivity Profile
INORGANIC NITRITES are oxidizing agents. React with acids to form toxic nitrogen dioxide gas. A violent explosion occurs if an ammonium salt is melted with a nitrite salt [Von Schwartz 1918. p 299]. A mixture with potassium cyanide may cause an explosion [Pieters 1957. p. 30].
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Nitrite Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Portail Substances Chimiques | -- | webmestre-substances@ineris.fr | France | 6027 | 58 |
| AccuStandard Inc | -- | info@bester.nl | United States | 5300 | 76 |
| Supplier | Advantage |
|---|---|
| Portail Substances Chimiques | 58 |
| AccuStandard Inc | 76 |
14797-65-0(Nitrite)Related Search:
1of4