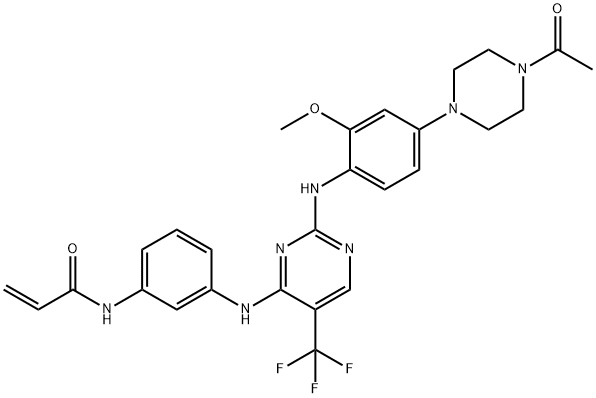
CO-1686
- CAS No.
- 1374640-70-6
- Chemical Name:
- CO-1686
- Synonyms
- CS-851;CNX-419;AVL-301;CO-1686;ROCILETINIB;Pyridostadin;CO-1686, >=98%;Clovis CO-1686;CO-1686/CO1686;CO-1686 (AVL-301)
- CBNumber:
- CB22666396
- Molecular Formula:
- C27H28F3N7O3
- Molecular Weight:
- 555.55
- MDL Number:
- MFCD26793864
- MOL File:
- 1374640-70-6.mol
- MSDS File:
- SDS
| Melting point | 202 - 205°C (dec.) |
|---|---|
| Density | 1.372±0.06 g/cm3(Predicted) |
| storage temp. | Amber Vial, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 13.23±0.70(Predicted) |
| color | Off-White |
| CAS DataBase Reference | 1374640-70-6 |
| FDA UNII | 72AH61702G |
| NCI Drug Dictionary | rociletinib |
| ATC code | L01EB05 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P270-P271-P280-P301+P312-P330-P302+P352-P321-P304+P340-P305+P351+P338-P332+P313-P362+P364-P337+P313-P403+P233-P405-P501 | |||||||||
| Risk Statements | 23-25-36 | |||||||||
| Safety Statements | 24 | |||||||||
| HS Code | 29335990 | |||||||||
| NFPA 704 |
|
CO-1686 price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 16244 | CO-1686 ≥98% | 1374640-70-6 | 1mg | $68 | 2024-03-01 | Buy |
| Cayman Chemical | 16244 | CO-1686 ≥98% | 1374640-70-6 | 5mg | $201 | 2024-03-01 | Buy |
| Cayman Chemical | 16244 | CO-1686 ≥98% | 1374640-70-6 | 10mg | $335 | 2024-03-01 | Buy |
| TRC | C633000 | CO1686 | 1374640-70-6 | 100mg | $275 | 2021-12-16 | Buy |
| ChemScene | CS-1631 | Rociletinib 99.59% | 1374640-70-6 | 100mg | $250 | 2021-12-16 | Buy |
CO-1686 Chemical Properties,Uses,Production
Anti-cancer drugs
On May 20, 2014, Clovis Oncology announced that the US FDA had granted its test drug CO-1686 for breakthrough treatment drug qualification, as a second line, single administrated drug for the treatment of EFGR mutation non-small cell lung cancer (NSCLC) of the T790M mutation patients. The awarding for this breakthrough therapeutic drug eligibility was based on the efficacy and safety results of Co-1686 in an ongoing Phase 1/2 study. Data of related study have shown that CO-1686 is a third-generation EGFR inhibitor, being used for the treatment of non-small cell lung cancer, overcoming the drug resistance generated from the EGFR T790M mutations, and has excellent efficacy and tolerability on NSCLC with T790M + EGFR mutations. Its major market competitor, the third-generation EGFR inhibitor, AZD9291 (AstraZeneca) has also gained FDA's groundbreaking drug eligibility.
CO-1686 is a novel, oral-administrated, targeted covalent (irreversible) inhibitor of the epidermal growth factor receptor (EGFR) mutations, being able to suppress key activation mutations and T790 drug-resistant mutations, leaving the wild-type EGFR signal unused. This drug was developed for the treatment of NSCLC patients carrying initially activated EGFR mutations and major resistant mutant T790M.
The above information is compiled and edited by Xiao Nan of Chemicalbook.
Biological activity
Rociletinib (CO-1686, AVL-301) is an irreversible, mutation-selective EGFR inhibitor that targets EGFRL858R/T790M and EGFRWT with a Ki of 21.5 nM and 303.3 nM, respectively. Phase 2.
Target: EGFR (L858R/T790M) EGFR (wt)
IC50: 21.5 nM (Ki) 303.3 nM (Ki)
In vitro study
CO-1686 inhibited the p-EGFR in EGFR-expressing cells with an IC50 ranging from 62 to 187 nM while inhibiting EGFR phosphorylation. In three WT EGFR-expressing cells, the IC50 is larger than 2,000 nM. CO-1686 can selectively inhibit the growth of mutant EGFR expressing NSCLC cells with a GI50 ranging from 7 to 32 nM while inducing apoptosis. The CO-1686-resistant NSCLC cell line exhibited a signal for epithelial-mesenchymal transition and increased susceptibility to AKT inhibitors.
In vivo studies
In all EGFR mutant models, as well as in transgenic mice expressing human EGFRL858R-and EGFRL858R/T790M, CO-1686 caused significant tumor growth inhibition in a dose-dependent manner.
Synthesis method
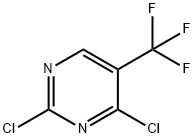
5-fluoro-2-nitroanisole was condensed with piperazine, acetylated and reduced to give 1-(3-methoxy-4-aminophenyl)-4-acetylpiperazine (4) In addition, use 2, 4-dichloro-5-trifluoro methyl pyrimidine to undergo condensation with 3-nitroaniline, reduction and amidation to obtain N-[3-(2-Chloro-5-trifluoromethyl-pyrimidin-4-amino) phenyl] acrylamide (7). 4 and 7 were condensed to give the EGFR inhibitor, the anticancer drug CO-1686 with a total yield of about 71% (based on 2, 4-dichloro-5-trifluoromethyl pyrimidine).
Reference: Synthesis of CO-1686 [J]. Chinese Pharmaceutical Industry, 2014, 45 (8): 710-713.
OF: (1) Lai Yisheng, male, professor, doctoral supervisor, engaged in anti-inflammatory and anti-tumor drugs studied. (2) Zhang Shan, female, graduate students, professional direction: medicinal chemistry.
Description
CO-1686 is an irreversible kinase inhibitor that specifically targets mutant forms of the epidermal growth factor receptor (EGFR) including T790M (Ki = 21.5 nM) with significantly reduced activity at the wild-
Uses
CO 1686 is seen as an irreversible epidermal growth factor receptor (EFGR1) kinase inhibitor.
target
L858R/T790M mutant EGFR
References
[1] walter a o, tjin r, haringsma h, et al. co-1686, an orally available, mutant-selective inhibitor of the epidermal growth factor receptor (egfr), causes tumor shrinkage in non-small cell lung cancer (nsclc) with t790m resistance mutations. mol cancer ther, 2011, 10(11 suppl).
[2] walter a o, sjin r t t, haringsma h j. discovery of a mutant-selective covalent inhibitor of egfr that overcomes t790m-mediated resistance in nsclc.
CO-1686 Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Jurong Coupling Biotechnology Co., Ltd. | 13656108824 | coupling278191416@hotmail.com | CHINA | 184 | 58 |