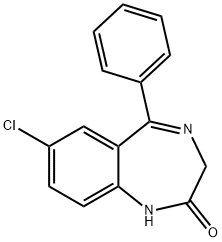
Nordazepam
- CAS No.
- 1088-11-5
- Chemical Name:
- Nordazepam
- Synonyms
- NORDIAZEPAM;DESMETHYLDIAZEPAM;NDZ;NDD;Dmdz;A 101;Madar;Nordaz;Stilny;Calmday
- CBNumber:
- CB2292432
- Molecular Formula:
- C15H11ClN2O
- Molecular Weight:
- 270.71
- MDL Number:
- MFCD00063440
- MOL File:
- 1088-11-5.mol
| Melting point | 208-210°C |
|---|---|
| Boiling point | 453.1±45.0 °C(Predicted) |
| Density | 1.2576 (rough estimate) |
| refractive index | 1.6330 (estimate) |
| Flash point | 11 °C |
| storage temp. | 2-8°C |
| solubility | DMSO: >10mg/mL |
| pka | pKa 3.5 12.0 (5%MeOH in H2O t=20 I=0.15) (Uncertain) |
| form | neat &_& powder |
| color | white to light tan |
| LogP | 2.930 |
| EWG's Food Scores | 1 |
| FDA UNII | 67220MCM01 |
| NIST Chemistry Reference | Nordazepam(1088-11-5) |
| ATC code | N05BA16 |
| EPA Substance Registry System | 2H-1,4-Benzodiazepin-2-one, 7-chloro-1,3-dihydro-5-phenyl- (1088-11-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 22-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 7-16-36/37-45 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DF1750000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2933910000 | |||||||||
| Toxicity | LD50 in mice (mg/kg): 2750 orally, >400 i.p. (Randall); LD50 in mice, rats (mg/kg): 1300, >5200 orally (company communication) | |||||||||
| NFPA 704 |
|
Nordazepam Chemical Properties,Uses,Production
Chemical Properties
Off-White Solid
Originator
Madar,Ravizza, Italy ,1973
Uses
The primary metabolite of Diazepam. A ligand for the GABAA receptor benzodiazepine modulatory site. Anxiolytic. Controlled substance (depressant).
Uses
The primary metabolite of diazepam. A ligand for the GABAA receptor benzodiazepine modulatory site. This is a controlled drug precursor therefore a liscence may be required for purchase
Definition
ChEBI: A 1,4-benzodiazepinone having phenyl and chloro substituents at positions 5 and 7 respectively; it has anticonvulsant, anxiolytic, muscle relaxant and sedative properties but is used primarily in the treatment of anxiety.
Manufacturing Process
A solution of 3.1 g of (2-benzoyl-4-chlorophenyl-carbamoylmethyl)carbamic acid benzyl ester in 30 cc of 20% hydrobromic acid in glacial acetic acid was stirred for 45 minutes at room temperature. On addition of 175 cc of anhydrous ether, a gummy solid precipitated. After several minutes the ether solution was decanted. The resultant 5-chloro-2-glycylaminobenzophenone was not isolated, but about 155 cc of ether was added to the residue and after chilling in an ice bath, 10% sodium hydroxide was added until the mixture was alkaline. The ether layer was then separated, washed twice with water and dried over sodium sulfate. After filtration, the ether solution was concentrated to dryness in vacuo. The residue was crystallized from benzene to yield 7-chloro-5-phenyl-3H-1,4-benzodiazepin-2(1H)-one.
Therapeutic Function
Tranquilizer
Synthesis Reference(s)
Journal of Heterocyclic Chemistry, 25, p. 459, 1988 DOI: 10.1002/jhet.5570250220
The Journal of Organic Chemistry, 26, p. 4936, 1961 DOI: 10.1021/jo01070a038