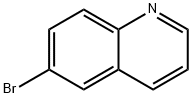
6-Bromoquinoline
- CAS No.
- 5332-25-2
- Chemical Name:
- 6-Bromoquinoline
- Synonyms
- 6-Bromoquinoline,97%;NSC 3996;6-BromoquinoL;6-Br-quinoline;6-bromo-quinolin;6-BROMOQUINOLINE;6-BROMOOQUINOLINE;Quinoline, 6-bromo-;TIMTEC-BB SBB001559;6-Bromoquinoline>
- CBNumber:
- CB2314673
- Molecular Formula:
- C9H6BrN
- Molecular Weight:
- 208.05
- MDL Number:
- MFCD00024023
- MOL File:
- 5332-25-2.mol
- MSDS File:
- SDS
| Melting point | 19°C |
|---|---|
| Boiling point | 116 °C / 6mmHg |
| Density | 1.538 g/mL at 25 °C |
| refractive index | n20/D 1.663 |
| Flash point | 19 °C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Soluble in acetone, acetonitrile, dichloromethane, ethyl acetate and THF. |
| pka | 4.18±0.10(Predicted) |
| form | Oil |
| InChIKey | IFIHYLCUKYCKRH-UHFFFAOYSA-N |
| CAS DataBase Reference | 5332-25-2(CAS DataBase Reference) |
| FDA UNII | KS7HD5UJ94 |
| NIST Chemistry Reference | 6-Bromo quinoline(5332-25-2) |
| EPA Substance Registry System | Quinoline, 6-bromo- (5332-25-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H318-H335 | |||||||||
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-41-37/38-22-20/21/22 | |||||||||
| Safety Statements | 26-37/39-39-36 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29334900 | |||||||||
| NFPA 704 |
|
6-Bromoquinoline price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 697893 | 6-Bromoquinoline 97% | 5332-25-2 | 1g | $32.6 | 2022-05-15 | Buy |
| Sigma-Aldrich | 697893 | 6-Bromoquinoline 97% | 5332-25-2 | 5g | $113 | 2022-05-15 | Buy |
| TCI Chemical | B2015 | 6-Bromoquinoline >95.0%(GC) | 5332-25-2 | 1g | $22 | 2024-03-01 | Buy |
| TCI Chemical | B2015 | 6-Bromoquinoline >95.0%(GC) | 5332-25-2 | 5g | $63 | 2024-03-01 | Buy |
| Alfa Aesar | H55111 | 6-Bromoquinoline, 97% | 5332-25-2 | 1g | $38.65 | 2024-03-01 | Buy |
6-Bromoquinoline Chemical Properties,Uses,Production
Bromoquinoline
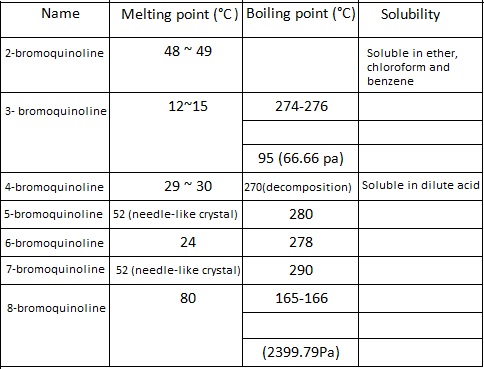
There are seven positional isomers of bromoquinoline and their main properties are listed below:
Application and synthetic method
3-bromo-quinoline is reacted with mixed acid for generating 3-bromo-5-nitro-quinoline, which heats together with potassium permanganate for being oxidation into 5-bromo-2, 3-pyridine dicarboxylic acid.
6-bromo-quinoline is heated together with nitric acid to generate 6-bromo-8-nitro quinolone with further reaction with potassium permanganate for being oxidized to 2, 3-pyridinedicarboxylic acid.
2-bromo-quinolien can be synthesized through the reaction between 2-hydroxy quinoline and phosphorus pentabromide.
Quinoline perbromide is heated at 180 °C for generating 3-bromo-quinoline.
From the heating between 4-hydroxy quinoline and phosphorus pentabromide, or from the diazotization reaction via 4-aminoquinoline to generate 4-bromo-quinoline;
5-bromo-quinoline is synthesize by the heating reaction between m-bromo-aniline, glycerol, m-bromo nitrobenzene and concentrated sulfuric acid, or from the diazotization reaction of 5-aminoquinoline.
6-bromo-quinoline can be synthesized from the heating of bromoaniline, glycerol, concentrated sulfuric acid, and p-bromo-nitrobenzene.
7-bromo-quinoline can be synthesized from the diazotization reaction of 7-aminoquinoline.
8-bromo-quinoline can be synthesized from the heating reaction of o-bromo-aniline, glycerol, concentrated sulfuric acid and o-bromo nitrobenzene.
application: as organic synthesis reagents.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Chemical Properties
Thick Oil
Uses
6-Bromoquinoline is used as fine chemical and pharmaceutical intermediate, used as the coupling reagent.
Synthesis
4-Bromobenzenamine (25 g, 145.32 mmol, 1.00 equiv), sodium 3-nitrobenzenesulfonate (55.5 g, 246.64 mmol, 1.70 equiv), propane-1,2,3-triol (50.8 g, 551.63 mmol, 3.80 equiv), and sulfuric acid (170 mL, 70%) were placed into a 500-mL round-bottom flask. The resulting solution was stirred overnight at 140° C. The pH value of the solution was adjusted to with 10% aqueous sodium hydroxide. The resulting solution was extracted with 5*150 mL of ethyl acetate. The organic layers were combined and dried over anhydrous sodium sulfate, and concentrated in vacuo. The residue was purified by silica gel column chromatography eluting with ethyl acetate/petroleum ether (1:50). This resulted in 17.2 g (42%) of 6-bromoquinoline as yellow oil.
6-Bromoquinoline Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| China Synchem Technology Co.,Ltd. | +86-0552-4929304 +86-18055277008 | wangxuhui1985@126.com | China | 55 | 55 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6710 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 1696 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
View Lastest Price from 6-Bromoquinoline manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | 6-Bromoquinoline
5332-25-2
|
US $5.00 / kg | 1kg | 99.92% | 50000tons | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-09 | 6-Bromoquinoline
5332-25-2
|
US $5.00-0.10 / KG | 1KG | 98% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2024-02-23 | 6-Bromoquinoline
5332-25-2
|
US $32.00-266.00 / g | 25g | 0.97 | 25kg | Shanghai UCHEM Inc. |
-

- 6-Bromoquinoline
5332-25-2
- US $5.00 / kg
- 99.92%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- 6-Bromoquinoline
5332-25-2
- US $5.00-0.10 / KG
- 98%
- Henan Fengda Chemical Co., Ltd
-

- 6-Bromoquinoline
5332-25-2
- US $32.00-266.00 / g
- 0.97
- Shanghai UCHEM Inc.