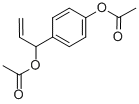
ChemicalBook >> CAS DataBase List >>ACETOXYCHAVICOL ACETATE, D/L-1''-
ACETOXYCHAVICOL ACETATE, D/L-1''-
- CAS No.
- 52946-22-2
- Chemical Name:
- ACETOXYCHAVICOL ACETATE, D/L-1''-
- Synonyms
- Galangal acetate;1'-Acetoxychavicol acetate;DL-1''-ACETOXYCAVICOL ACETATE;D,L-1'-Acetoxychavicol acetate;D,L-1'-ACETOXYCHAVICOL ACETATE;D/L-1''-ACETOXCHAVICOL ACETATE;AcetoXychvicol acetate, D/L-1''-;ACETOXYCHAVICOL ACETATE, D/L-1''-;ACETOXYCHAVICOL ACETATE, D/L-1''-;(1S)-1-[4-(acetyloxy)phenyl]prop-2-en-1-yl acetate
- CBNumber:
- CB2442536
- Molecular Formula:
- C13H14O4
- Molecular Weight:
- 234.25
- MDL Number:
- MFCD01632488
- MOL File:
- 52946-22-2.mol
- MSDS File:
- SDS
Last updated:2023-05-25 18:01:03
| storage temp. | Store at -20°C |
|---|---|
| solubility | DMF: 14 mg/mL,DMSO: 20 mg/mL,Ethanol: 20 mg/mL,Ethanol:PBS(pH 7.2) (1:1): 0.5 mg/mL |
| LogP | 1.900 (est) |
| FDA UNII | SQV3080A20 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H335-H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
ACETOXYCHAVICOL ACETATE, D/L-1''- price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHL82458 | 1′-Acetoxychavicol acetate phyproof? Reference Substance | 52946-22-2 | 10MG | $490 | 2023-01-07 | Buy |
| Cayman Chemical | 19649 | D,L-1′-Acetoxychavicol Acetate ≥95% | 52946-22-2 | 1mg | $32 | 2022-04-27 | Buy |
| Cayman Chemical | 19649 | D,L-1′-Acetoxychavicol Acetate ≥95% | 52946-22-2 | 5mg | $134 | 2022-04-27 | Buy |
| Cayman Chemical | 19649 | D,L-1′-Acetoxychavicol Acetate ≥95% | 52946-22-2 | 10mg | $251 | 2022-04-27 | Buy |
| Cayman Chemical | 19649 | D,L-1′-Acetoxychavicol Acetate ≥95% | 52946-22-2 | 25mg | $589 | 2022-04-27 | Buy |
ACETOXYCHAVICOL ACETATE, D/L-1''- Chemical Properties,Uses,Production
Description
D,L-1′-Acetoxychavicol acetate is a natural compound first isolated from the rhizomes of ginger-like plants. It has a wide array of cellular effects that may be largely explained by its ability to inhibit exportin 1 and prevent the export of proteins from the nucleus. In this way, D,L-1′-acetoxychavicol acetate reduces several intracellular signaling pathways, including NF-κB, impacting inflammation, cancer, viral replication, and other processes.
Definition
ChEBI: An acetate ester that is chavicol acetate substituted by an acetoxy group at position 1'.
ACETOXYCHAVICOL ACETATE, D/L-1''- Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 30)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9702 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Nanjing Vital Chemical Co., Ltd. | Please Send Email 18652950785 | chemweiao@163.com | China | 4388 | 65 |
| Sichuan Wei Keqi Biological Technology Co., Ltd. | 028-81700200 18116577057 | 3003855609@qq.com | China | 7892 | 56 |
| Finetech Industry Limited | 027-87465837 19945049750 | sales@finetechnology-ind.com | China | 9664 | 58 |
| Shenzhen SUNGENING Bio-Medical Co., Ltd. | 0755-28967200 13631290199 | wxf@sungening.com | China | 9506 | 60 |
52946-22-2(ACETOXYCHAVICOL ACETATE, D/L-1''-)Related Search:
D/L-1''-ACETOXCHAVICOL ACETATE
D,L-1'-ACETOXYCHAVICOL ACETATE
ACETOXYCHAVICOL ACETATE, D/L-1''-
1'-Acetoxychavicol acetate
DL-1''-ACETOXYCAVICOL ACETATE
(S)-4-(Acetyloxy)-alpha-ethenylbenzenemethanol acetate
Galangal acetate
AcetoXychvicol acetate, D/L-1''-
(1S)-1-[4-(acetyloxy)phenyl]prop-2-en-1-yl acetate
ACETOXYCHAVICOL ACETATE, D/L-1''-
D,L-1'-Acetoxychavicol acetate
52946-22-2
fine chemicals, specialty chemicals, intermediates, electronic chemical, organic synthesis