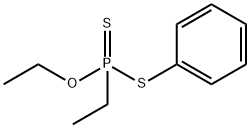
FONOFOS
- CAS No.
- 944-22-9
- Chemical Name:
- FONOFOS
- Synonyms
- 10 G;CAPFOS;N-2790;oms410;Captos;FONOFOS;OMS 410;Dyfonat;DYFONATE;FONOPHOS
- CBNumber:
- CB2463599
- Molecular Formula:
- C10H15OPS2
- Molecular Weight:
- 246.33
- MDL Number:
- MFCD00055421
- MOL File:
- 944-22-9.mol
| Melting point | 32℃ |
|---|---|
| Boiling point | bp0.1 130° |
| Density | d2525 1.16 |
| vapor pressure | 2.8 x 10-2 Pa (25 °C) |
| refractive index | nD30 1.5883 |
| Flash point | 2 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly) |
| Water Solubility | 13 mg l-1(22 °C) |
| Merck | 13,4258 |
| BRN | 1958949 |
| Exposure limits | NIOSH REL: 0.1 mg/m3; ACGIH TLV: 0.1 mg/m3 |
| EWG's Food Scores | 4-5 |
| FDA UNII | P4VT8081QO |
| Pesticides Freedom of Information Act (FOIA) | Fonofos |
| EPA Substance Registry System | Fonofos (944-22-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS06,GHS09,GHS02,GHS07 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300+H310+H330-H410-H225-H302+H332-H319-H412 |
| Precautionary statements | P260-P264-P273-P280-P284-P301+P310-P210-P305+P351+P338 |
| Hazard Codes | T+,N,Xn,F |
| Risk Statements | 27/28-50/53-36-20/21/22-11-52/53-23 |
| Safety Statements | 28-36/37-45-60-61-16-26 |
| RIDADR | 3018 |
| WGK Germany | 3 |
| RTECS | TA5950000 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Toxicity | LD50 in mice (mg/kg): 14.0 orally, 4.8 i.p. (Lee); LD50 in rats (mg/kg): 3-17 orally, 147 dermally (Ben-Dyke) |
FONOFOS Chemical Properties,Uses,Production
Description
Fonofos is a pale yellow liquid with a pungent,mercaptan-like odor. Molecular weight=246.34; Boilingpoint=130℃ at 0.1 mm; Freezing/Melting point=30℃;Vapor pressure=0.0003 mmHg at 20℃; Flashpoint=94℃ (cc). Hazard Identification (based on NFPA-704 M Rating System): Health 4, Flammability 1,Reactivity 0. Insoluble in water.
Chemical Properties
Yellow liquid. Insoluble in water; miscible with organic solvents.
Chemical Properties
Fonofos is a pale yellow liquid with a pun gent, mercaptan-like odor.
Chemical Properties
Fonofos is a highly toxic compound. It is sparingly soluble in water, but soluble in acetone, ethanol, xylene, and kerosene. It has been grouped by the US EPA under RUP and hence requires special handling by qualifi ed, certifi ed, and trained workers. Fonofos was used as a soil insecticide, which resulted in its direct release to the environment. It was primarily used on corn crops, sugar cane, peanuts, tobacco, turf, and some vegetable crops. It controls aphids, corn borer, corn root-worm, corn wire-worm, cutworms, white grubs, and some maggots. Formulations of fonofos include granular, microgranular, emulsifi able concentrate, suspension concentrate, microcapsule suspension, and for seed treatment
Uses
Fonofos is used to control soil insects in a number of crops and in turf.
Uses
Soil insecticide used to control rootworms, wireworms, crickets and similar crop pests in vegetables, sorghum, ornamentals, cereals, maize, vines, olives, sugar beet, sugarcane, potatoes, groundnuts, tobacco, turf and fruit crops.
Uses
Soil insecticide.
Definition
ChEBI: Fonofos is an organic thiophosphate and an organothiophosphate insecticide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an EC 3.1.1.8 (cholinesterase) inhibitor and an agrochemical.
General Description
Light-yellow liquid with a pungent mercaptan-like odor. Used primarily as an insecticide for corn.
Air & Water Reactions
Very slightly soluble in water.
Reactivity Profile
Organothiophosphates, such as FONOFOS, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. It is sparingly soluble in water but soluble in acetone, ethanol, xylene, and kerosene.
Hazard
Cholinesterase inhibitor.
Health Hazard
Cholinesterase inhibitor; highly toxic byingestion and skin absorption; exhibitsacute, delayed, and chronic poisoning; toxicsymptoms include headache, blurred vision,pinpoint pupils, salivation, tearing, musclespasms, vomiting, abdominal pain, diarrhea,seizure, shortness of breath, and respiratoryarrest; ingestion of 0.5–29 can cause deathto adult humans; median lethal dose (oral)in rat reported in the literature varies at3–25 mg/kg; bioaccumulation potential ofthis toxicant is expected to be high becauseof high K ow (8000):
LD50 oral (rat): 3–25 mg/kg
LD50 oral (dog): 3.3 mg/kg
LD50 skin (rabbit): 25 mg/kg
LD50 skin (rat): 147 mg.
Health Hazard
Fonofos is highly toxic like many other organophosphate pesticides to humans and animals. Exposure to fonofos induces clinical signs of toxicity with typical symptoms of poisoning and cholinesterase inhibition. Accidental ingestion of fonofos by occupational workers results in signs and symptoms of acute intoxication, including muscarinic, nicotinic, and CNS manifestations. Symptoms of fonofos poisoning is a delayed process and occur within a few minutes to 12 h after exposure. Early symptoms of poisoning include, but are not limited to, blurred vision, pinpoint pupils, headache, dizziness, depression, tremors, salivation, diarrhea, and labored breathing. Skin absorption of fonofos causes sweating and muscle twitching, while eye contact leads to severe tearing, pain, and blurred vision. Prolonged exposures to high concentrations of fonofos lead to respiratory failure and death. There are no reports indicating that fonophos is mutagenic, teratogenic, or carcinogenic in animals and humans. Fonofos registration was cancelled in 1999, therefore, it is not considered an environmental contaminant of concern at the present time.
Health Hazard
FONOFOS is a cholinesterase inhibitor. It can cause severe symptoms and death from respiratory arrest.
Fire Hazard
When FONOFOS is heated to decomposition, FONOFOS can emit highly toxic fumes of phosphorus oxides.
Agricultural Uses
Insecticide: It is a soil organophosphate insecticide primarily used on corn. It was also used on maize, cereals, sorghum, fruit, olives, potatoes, sugar cane, peanuts, tobacco, turf, and some vegetable crops. It controls aphids, corn borer, corn rootworm, corn wireworm, cutworms, white grubs, symphylins, and other soil pests including some maggots. It was available in granular, microgranular, emusifiable concentrate, suspension concentrate, microcapsule suspension, and seed treatment. Not registered for use in the U.S. or in EU countries. There are 16 global suppliers.
Trade name
CAPFOS®; CUDGEL®; DIFONATE®; DYFONATE®[C]; DYPHONATE®; DOUBLE DOWN®; STAUFFER-2790
Safety Profile
Poison by ingestion and skin contact. An insecticide. When heated to decomposition it emits very toxic fumes of POx and SOx.
Potential Exposure
A potential danger to those involved in the manufacture, formulation and application of this soil insecticide which is used for control of corn rootworms, wireworms, cutworms, symphylins, and other soil pests. Incompatibilities: Strong acids, alkalies.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Keepvictim quiet and maintain normal body temperature. Effectsmay be delayed; keep victim under observation.
Environmental Fate
Soil. The half-life for fonofos in soil incubated in the laboratory under aerobic conditions ranged was 25 days (Lichtenstein et al., 1977). In field soils, the half-lives for fonofos ranged from 24 to 102 days (Kiigemgi and Terriere, 1971; Schulz and Lichtenstein, 1971; Mathur et al., 1976; Talekar et al., 1977).
Plant. In plants, fonofos is oxidized to the phosphonothioate (Hartley and Kidd, 1987). Oat plants were grown in two soils treated with [14C]fonofos. Most of the residues remained bound to the soil. Less than 2% of the applied [14C]fonofos was re
Chemical/Physical. The hydrolysis half-lives of fonofos in a sterile 1% ethanol/water solution at 25°C and pH values of 4.5, 5.0, 6.0, 7.0 and 8.0, were 87, 50, 41, 22 and 6.9 weeks, respectively (Chapman and Cole, 1982). Emits toxic nitrogen and
Metabolic pathway
The metabolism of fonofos in plants and animals follows similar pathways, with both activation and degradative steps occurring as primary metabolic events. The former involves oxidative desulfuration to the active acetylcholinesterase inhibitor fonofos oxon. The oxon is hydrolysed rapidly in most biological systems and the resulting thiophenol is very rapidly metabolised via S-methylation, thiooxidation, ring hydroxylation and conjugation.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool area.
Shipping
UN2783 Organophosphorus pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Degradation
Fonofos is hydrolysed in acidic and alkaline media (PM).
Incompatibilities
Strong acids, alkalies.
Waste Disposal
This phosphono compound is reported to be satisfactorily decomposed by hypochlorite. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
FONOFOS Preparation Products And Raw materials
Raw materials
1of3