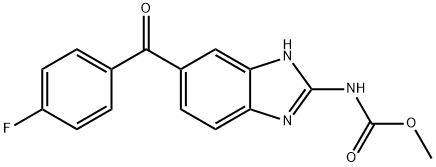
Flubendazole
- CAS No.
- 31430-15-6
- Chemical Name:
- Flubendazole
- Synonyms
- FLUBENDAZOL;Flubendazole Polymorph B;R-17899;R 17889;FLUMOXAL;FLUBENOL;FLUVERNAL;FLUMOXANE;fluvermal;Flubenvet
- CBNumber:
- CB2669630
- Molecular Formula:
- C16H12FN3O3
- Molecular Weight:
- 313.28
- MDL Number:
- MFCD00871999
- MOL File:
- 31430-15-6.mol
| Melting point | 290°C |
|---|---|
| Density | 1.3720 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, in alcohol and in methylene chloride |
| pka | 10.66±0.10(Predicted) |
| color | White to Off-White |
| Merck | 14,4118 |
| CAS DataBase Reference | 31430-15-6(CAS DataBase Reference) |
| FDA UNII | R8M46911LR |
| ATC code | P02CA05 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361 | |||||||||
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501 | |||||||||
| Risk Statements | 22 | |||||||||
| Safety Statements | 44 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DD6497500 | |||||||||
| HS Code | 29339900 | |||||||||
| Toxicity | LD50 in mice, rats, guinea pigs (mg/kg): >2560 orally (Thienpont) | |||||||||
| NFPA 704 |
|
Flubendazole price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | Y0000138 | Flubendazol European Pharmacopoeia (EP) Reference Standard | 31430-15-6 | y0000138 | $150 | 2024-03-01 | Buy |
| Sigma-Aldrich | 34091 | Flubendazol VETRANAL , analytical standard | 31430-15-6 | 100mg | $206 | 2024-03-01 | Buy |
| TCI Chemical | F0825 | Flubendazole >98.0%(HPLC)(T) | 31430-15-6 | 5g | $59 | 2024-03-01 | Buy |
| TCI Chemical | F0825 | Flubendazole >98.0%(HPLC)(T) | 31430-15-6 | 25g | $191 | 2024-03-01 | Buy |
| Cayman Chemical | 26064 | Flubendazole | 31430-15-6 | 5g | $57 | 2024-03-01 | Buy |
Flubendazole Chemical Properties,Uses,Production
Description
Flubendazole is a benzimidazole carbamate anthelmintic. It completely eliminates larvae in a mouse model of A. cantonensis infection and exhibits a mean larval reduction of 100% in a T. spiralis infection model when administered at doses of 5 and 50 mg/kg per day, respectively. Flubendazole inhibits mammalian tubulin polymerization (IC50 = 2.5 μM) and inhibits binding of [3H]mebendazole to H. contortus L3 larval tubulin (IC50 = 0.17 μM). Flubendazole also inhibits the proliferation of BT-549, SK-BR-3, MDA-MB-231, and MCF-7 breast cancer cells (IC50s = 0.72, 1.51, 1.75, and 5.51 μM, respectively) and reduces tumor growth in an MDA-MB-231 mouse xenograft model when administered at a dose of 25 mg/kg.
Chemical Properties
White Solid
Originator
Fluvermal,Janssen-Le Brun,France,1980
Uses
An insectocidal agent.
Uses
An anthelmintic.
Uses
VETRANAL? analytical standard can be used in the liquid chromatography coupled to mass spectrometry (LC-MS/MS) procedure, performed for the analysis of macrocyclic lactones in milk.
Definition
ChEBI: A member of the class of mebendazole in which the benzoyl group is replaced by a p-fluorobenzoyl group. A broad-spectrum anthelmintic, it is used, particularly in veterinary medicine, for the treatment of nematodal infections.
Manufacturing Process
To a stirred and cooled (ice bath) suspension of 25 parts of aluminum chloride
in 52 parts of fluorobenzene is added dropwise a solution of 27.5 parts of 4-
chloro-3-nitrobenzoyl chloride in 52 parts of fluorobenzene. Upon completion,
stirring is continued overnight at room temperature. The reaction mixture is
poured onto water and the product is extracted with methylene chloride. The
extract is washed successively with sodium hydrogen carbonate solution and
water, dried, filtered and evaporated in vacuo. The solid residue is crystallized
from 2-propanol, yielding 4-chloro-4'-fluoro-3-nitrobenzophenone; MP 97.9°C.
A mixture of 24.5 parts of 4-chloro-4'-fluoro-3-nitrobenzophenone, 72 parts of
methanol, 13 parts of sulfolane and 3.12 parts of ammonia is heated in a
sealed tube for 20 hours at 120°C. To the reaction mixture is added
successively 50 parts of water and 25 parts of a diluted hydrochloric acid
solution and the whole is stirred and refluxed for 5 minutes. The reaction
mixture is cooled and the precipitated product is filtered off. It is washed with 2-propanol and recrystallized from 640 parts of toluene, yielding 4-amino-4'-
fluoro-3-nitrobenzophenone; MP 199°C.
A mixture of 14.5 parts of 4-amino-4'-fluoro-3-nitrobenzophenone, 160 parts
of methanol, 6 parts of concentrated hydrochloric acid solution and 0.5 part of
platinum oxide is hydrogenated at normal pressure and at room temperature.
After the calculated amount of hydrogen is taken up, hydrogenation is
stopped. The catalyst is filtered off and the filtrate is evaporated. The residue
is washed with 2-propanol and dried, yielding 3,4-diamino-4'-
fluorobenzophenone hydrochloride; MP 226°C to 230.5°C.
A mixture of 89 parts of S-methylisothiourea sulfate, 6.05 parts of methyl
chloroformate in 7 parts of water is cooled, and at a temperature of 5°C to
10°C, sodium hydroxide solution 25% is added until pH equals 8. Then there
are added successively 6.4 parts of acetic acid, 2.6 parts of sodium acetate
and 8.9 parts of 3,4-diamino-4'-fluorobenzophenone hydrochloride and the
whole is stirred while heating at 85°C for 45 minutes (during this reaction
time, water and 2-propanol is added). The precipitated product is filtered off,
washed with methanol and recrystallized from a mixture of 200 parts of acetic
acid and 80 parts of methanol, yielding methyl N-[5(6)-p-fluorobenzoyl-2-
benzimidazolyl] carbamate; MP > 260°C.
Therapeutic Function
Anthelmintic
General Description
Flubendazol is a broad spectrum anthelmintic, which belongs to the class of compounds known as benzimidazoles. It can be widely used in veterinary and human medicine. It mainly finds applications in deworming poultry, swine, dogs and cats.
Biochem/physiol Actions
Flubendazole, an antithelmintic, has been widely used in treating intestinal parasites. Additionally, Flubendazole has been reported to exert anticancer activities.
Flubendazole Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 | sales001@rokechem.com | China | 255 | 58 |
| Sigma Audley | +86-18336680971 +86-18126314766 | nova@sh-teruiop.com | China | 524 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from Flubendazole manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-29 | flubendazole
31430-15-6
|
US $0.00-0.00 / g | 1g | 99.99% | 20 tons | airuikechemical co., ltd. | |
 |
2024-03-16 | Flubendazole
31430-15-6
|
US $35.00-25.00 / kg | 1kg | 99.8% | 200tons/year | Sigma Audley | |
 |
2023-09-20 | Flubendazole
31430-15-6
|
US $0.00 / kg | 1kg | 0.99 | 20tons | Hebei Yanxi Chemical Co., Ltd. |
-

- flubendazole
31430-15-6
- US $0.00-0.00 / g
- 99.99%
- airuikechemical co., ltd.
-

- Flubendazole
31430-15-6
- US $35.00-25.00 / kg
- 99.8%
- Sigma Audley
-

- Flubendazole
31430-15-6
- US $0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.