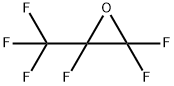
Hexafluoropropylene oxide
- CAS No.
- 428-59-1
- Chemical Name:
- Hexafluoropropylene oxide
- Synonyms
- HFPO;Perfluoropropylene oxide;Hexafluoropropene epoxide;trifluoro(trifluoromethyl)oxirane;2,2,3-Trifluoro-3-(trifluoromethyl)oxirane;NA 1956;(S) TRIMETHYLOXIRANE;Epoxyhexafluoropropane;HEXAFLUOROEPOXYPROPANE;Sevoflurane Impurity 2
- CBNumber:
- CB2675185
- Molecular Formula:
- C3F6O
- Molecular Weight:
- 166.02
- MDL Number:
- MFCD00005125
- MOL File:
- 428-59-1.mol
- MSDS File:
- SDS
| Melting point | -129°C |
|---|---|
| Boiling point | −42 °C(lit.) |
| Density | 1.67±0.1 g/cm3(Predicted) |
| vapor pressure | 680.85kPa at 25℃ |
| Water Solubility | 16.3μg/L at 22.7℃ |
| InChI | InChI=1S/C3F6O/c4-1(2(5,6)7)3(8,9)10-1 |
| InChIKey | PGFXOWRDDHCDTE-UHFFFAOYSA-N |
| SMILES | O1C(F)(C(F)(F)F)C1(F)F |
| LogP | 1.72 at 25℃ |
| CAS DataBase Reference | 428-59-1(CAS DataBase Reference) |
| FDA UNII | JPY12AP87N |
| NIST Chemistry Reference | Oxirane, trifluoro(trifluoromethyl)-(428-59-1) |
| EPA Substance Registry System | Oxirane, 2,2,3-trifluoro-3-(trifluoromethyl)- (428-59-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS02 |
|---|---|
| Signal word | Danger |
| Hazard statements | H314-H225-H290 |
| Precautionary statements | P501-P240-P210-P233-P234-P243-P241-P242-P264-P280-P370+P378-P390-P303+P361+P353-P301+P330+P331-P363-P304+P340+P310-P305+P351+P338+P310-P403+P235-P406-P405 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38-44 |
| Safety Statements | 7-26-37/39-38 |
| RIDADR | UN 1956 2.2 |
| WGK Germany | 3 |
| Hazard Note | Irritant |
| HazardClass | 2.2 |
Hexafluoropropylene oxide price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Oakwood | 003257 | Hexafluoropropene oxide | 428-59-1 | 25g | $50 | 2021-12-16 | Buy |
| SynQuest Laboratories | 2109-2-02 | Hexafluoropropene oxide 97% | 428-59-1 | 100g | $90 | 2021-12-16 | Buy |
| Oakwood | 003257 | Hexafluoropropene oxide | 428-59-1 | 100g | $100 | 2021-12-16 | Buy |
| SynQuest Laboratories | 2109-2-02 | Hexafluoropropene oxide 97% | 428-59-1 | 250g | $135 | 2021-12-16 | Buy |
| Oakwood | 003257 | Hexafluoropropeneoxide 97% | 428-59-1 | 500g | $290 | 2021-12-16 | Buy |
Hexafluoropropylene oxide Chemical Properties,Uses,Production
Uses
Hexafluoropropylene oxide is an important intermediate of organic fluorine material.HFPO is mainly used in the manufacture of high-quality fluorinated compounds.It is the main component of perfluor-vinyl ether(PPVE,PSVE,PFVE,PMVE) and the monomer of fluorinated surfactants and fluorinated ether oil.
General Description
Hexafluoropropylene oxide is a colorless odorless gas. Hexafluoropropylene oxide is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite. Its vapors are heavier than air. Hexafluoropropylene oxide can cause asphyxiation by the displacement of air. Exposure of the container to prolonged heat or fire may cause Hexafluoropropylene oxide to rupture violently and rocket.
Reactivity Profile
Hexafluoropropylene oxide is a chlorinated epoxide. Epoxides are highly reactive. They polymerize in the presence of catalysts or when heated. These polymerization reactions can be violent. Compounds in this group react with acids, bases, and oxidizing and reducing agents. They react, possibly violently with water in the presence of acid and other catalysts.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.
Flammability and Explosibility
Non flammable
Hexafluoropropylene oxide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Qingdao Dexin Chemical Co., Ltd | +8615553333686 | 15553333686@qq.com | China | 2983 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
View Lastest Price from Hexafluoropropylene oxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-26 | Hexafluoropropylene oxide
428-59-1
|
US $100.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2020-02-26 | Hexafluoropropylene oxide
428-59-1
|
US $0.00-0.00 / Kg | 1KG | 99.0%+ | 1000 tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2020-01-07 | Hexafluoropropylene oxide
428-59-1
|
US $1.00 / KG | 1KG | 99% | 20T | Shaanxi Dideu Medichem Co. Ltd |
-

- Hexafluoropropylene oxide
428-59-1
- US $100.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Hexafluoropropylene oxide
428-59-1
- US $0.00-0.00 / Kg
- 99.0%+
- Shaanxi Dideu Medichem Co. Ltd
-

- Hexafluoropropylene oxide
428-59-1
- US $1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd