ChemicalBook >> CAS DataBase List >>N-Hydroxyurethane
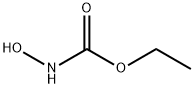
N-Hydroxyurethane
- CAS No.
- 589-41-3
- Chemical Name:
- N-Hydroxyurethane
- Synonyms
- NHU;SQ 16819;NSC-83629;NSC-71045;85.0%(GC&HYDROXYURETHAN;Hydroxyurethane;N-HYDROXYURETHAN;N-HYDROXYURETHANE;N-Hydroxyurethane>
- CBNumber:
- CB2725765
- Molecular Formula:
- C3H7NO3
- Molecular Weight:
- 105.09
- MDL Number:
- MFCD00002108
- MOL File:
- 589-41-3.mol
Last updated:2023-06-08 17:06:36
| Boiling point | 113-116 °C3 mm Hg(lit.) |
|---|---|
| Density | 1.3895 (rough estimate) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | -20°C |
| solubility | Diethyl Ether, Dimethyl Sulfoxide, Water |
| form | Oil |
| pka | 9.30±0.23(Predicted) |
| color | Yellow |
| BRN | 1747529 |
| CAS DataBase Reference | 589-41-3(CAS DataBase Reference) |
| FDA UNII | QWI3C4ASI7 |
| NIST Chemistry Reference | Carbamic acid, hydroxy-, ethyl ester(589-41-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332 | |||||||||
| Precautionary statements | P501-P261-P270-P271-P264-P280-P362+P364-P301+P312+P330-P302+P352+P312-P304+P340+P312 | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36/37/39 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | FB1750000 | |||||||||
| HS Code | 2928009090 | |||||||||
| Toxicity | LD50 intraperitoneal in rat: 800mg/kg | |||||||||
| NFPA 704 |
|
N-Hydroxyurethane price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 119474 | N-Hydroxyurethane | 589-41-3 | 5g | $246 | 2023-06-20 | Buy |
| Sigma-Aldrich | 119474 | N-Hydroxyurethane | 589-41-3 | 25g | $531 | 2022-05-15 | Buy |
| TCI Chemical | H0618 | N-Hydroxyurethane >85.0%(GC)(N) | 589-41-3 | 1g | $31 | 2024-03-01 | Buy |
| TCI Chemical | H0618 | N-Hydroxyurethane >85.0%(GC)(N) | 589-41-3 | 5g | $109 | 2024-03-01 | Buy |
| TRC | H991050 | Hydroxyurethane | 589-41-3 | 25g | $395 | 2021-12-16 | Buy |
N-Hydroxyurethane Chemical Properties,Uses,Production
Chemical Properties
Yellow Oil
Uses
Reactant involved in:
- Synthesis of molecules used for intermolecular Sharpless aminohydroxylation reactions
- Intermolecular ortho-C-H amidation of anilides
- Cinchona alkaloid-catalyzed asymmetric cycloaddition
- Allylic arylation
Uses
Hydroxyurethane is a metabolite of carcinogenic Urethane (U825300).
Biochem/physiol Actions
N-Hydroxyurethane causes the chromosomal fragmentation at millimolar concentrations and cell toxicity in cultured normal human leukocytes.
N-Hydroxyurethane Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 109)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Alfa Chemistry | info@alfa-chemistry.com | United States | 2344 | 58 | |
| Aston Chemical | 13000000000 | sales@astonchem.com | United States | 1634 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 | cherry@crovellbio.com | China | 18458 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Nanjing Bicbiotechnology Co., Ltd | +86-2552131256 +86-18251840740 | sales@bicbiotech.com | China | 6000 | 58 |
589-41-3(N-Hydroxyurethane)Related Search:
n-hydroxyethylcarbamate
NSC-71045
NSC-83629
SQ 16819
N-HYDROXYURETHAN
N-HYDROXYURETHANE
N-HYDROXYURETHAN 94+%
Ethyl hydroxycarbamate 97%
HYDROXYURETHAN
N-hydroxycarbamic acid ethyl ester
N-Hydroxycarbamic acid ethyl
ETHYL HYDROXYCARBAMATE
ETHYL N-HYDROXYCARBAMATE
HYDROXYCARBAMIC ACID ETHYL ESTER
Ethylester kyseliny N-hydroxykarbaminove
Ethyl Hydroxycarbamate
Hydroxycarbamic Acid Ethyl Ester
N-Hydroxyurethane
ethylesterkyselinyn-hydroxykarbaminove
hydroxy-carbamicaciethylester
Hydroxyurethane
N-Carbethoxyhydroxylamine
NHU
N-Hydroxy ethyl carbamate
N-Hydroxyurethane>
85.0%(GC&
Carbamic acid, N-hydroxy-, ethyl ester
589-41-3
HONHCOOCH2CH3
Hydroxylamines
Hydroxylamines (N-Substituted)
Organic Building Blocks
Nitrogen Compounds
Protected Amines
Building Blocks
Amines
Mutagenesis Research Chemicals
Aliphatics
Hydroxylamines
Hydroxylamines (N-Substituted)