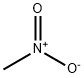
Nitromethane
- CAS No.
- 75-52-5
- Chemical Name:
- Nitromethane
- Synonyms
- CH3NO2;NM;methane,-nitro-;NITOMETHANE;NITROCARBOL;XJJW;NM-55;Nitrofuel;Nitrometan;NITROMETANO
- CBNumber:
- CB2753821
- Molecular Formula:
- CH3NO2
Lewis structure
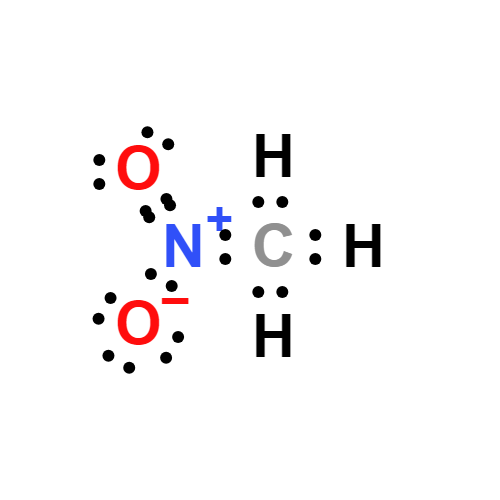
- Molecular Weight:
- 61.04
- MDL Number:
- MFCD00007400
- MOL File:
- 75-52-5.mol
- MSDS File:
- SDS
| Melting point | ?29 °C (lit.) |
|---|---|
| Boiling point | 101.2 °C (lit.) |
| Density | 1.127 g/mL at 25 °C (lit.) |
| vapor density | 2.1 (vs air) |
| vapor pressure | 27.3 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 95 °F |
| storage temp. | Flammables area |
| solubility | 105g/l |
| pka | 10.2(at 25℃) |
| form | Liquid |
| color | APHA: ≤10 |
| PH | 6.4 (0.6g/l, H2O, 20℃) |
| Relative polarity | 6.8 |
| Odor | Disagreeable fruity odor |
| PH Range | 6.4 at 0.01 g/l at 20 °C |
| Evaporation Rate | 1.39 |
| explosive limit | 7.3-63.0%(V) |
| Water Solubility | 9.5 g/100 mL (20 ºC) |
| λmax |
λ: 380 nm Amax: 1.00 λ: 386 nm Amax: 0.50 λ: 395 nm Amax: 0.20 λ: 400 nm Amax: 0.10 λ: 405 nm Amax: 0.05 λ: 430-700 nm Amax: 0.01 |
| Merck | 14,6611 |
| BRN | 1698205 |
| Henry's Law Constant | 2.24 at 20.00 °C, 3.61 at 30.00 °C, 5.40 at 40.00 °C, 7.97 at 50.00 °C (inert gas stripping, Bene? and Dohnal, 1999) |
| Exposure limits | NIOSH REL: IDLH 750 ppm; OSHA PEL: TWA 100 ppm (250 mg/m3); ACGIH TLV: TWA 20 ppm (adopted). |
| Dielectric constant | 22.7(Ambient) |
| InChIKey | LYGJENNIWJXYER-UHFFFAOYSA-N |
| CAS DataBase Reference | 75-52-5(CAS DataBase Reference) |
| EWG's Food Scores | 4-6 |
| NCI Dictionary of Cancer Terms | nM |
| FDA UNII | RU5WG8C3F4 |
| Proposition 65 List | Nitromethane |
| NIST Chemistry Reference | Methane, nitro-(75-52-5) |
| IARC | 2B (Vol. 77) 2000 |
| EPA Substance Registry System | Nitromethane (75-52-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226-H302+H332-H351-H361fd | |||||||||
| Precautionary statements | P201-P202-P210-P301+P312-P304+P340+P312-P308+P313 | |||||||||
| Hazard Codes | Xn,F,Xi | |||||||||
| Risk Statements | 5-10-22 | |||||||||
| Safety Statements | 41 | |||||||||
| RIDADR | UN 1261 3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | PA9800000 | |||||||||
| F | 3-10 | |||||||||
| Autoignition Temperature | 784 °F | |||||||||
| Hazard Note | Irritant/Flammable | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29042090 | |||||||||
| Toxicity | LC (in air) in guinea pigs: 1000 ppm; LD50 orally in mice: 1.44 g/kg (Weatherby) | |||||||||
| IDLA | 750 ppm | |||||||||
| NFPA 704 |
|
Nitromethane Chemical Properties,Uses,Production
Description
Nitromethane (75-52-5) is an explosive material that was originally manufactured for various applications including mining, construction, demolition, law enforcement, and military uses. However, due to threats of terrorism and increased attention to accident prevention, regulations concerning the transportation, storage, use, and transfer relating to explosives have steadily increased over the last few years and manufacturing limited.
Chemical Properties
Nitromethane is explosive and can be detonated by shock or heat (HSDB 1988) and the chemical can be made more sensitive to detonation through the presence of other chemicals, especially amines and acids. Nitromethane forms salts with inorganic bases and the dry salts are explosive.
Chemical Properties
Nitromethane is a highly flammable and explosive colorless liquid with a strong, disagreeable odor. Nitromethane is not explosive, but is used as industrial chemical for various purposes. Nitromethane can explode only in big quantity and in strong confinement. In combination with some further components, nitromethane is the important part of very strong, cap sensitive explosives. Therefore, nitromethane is an easy accessible precursor for preparation of strong home-made explosives.
Nitromethane is used as a stabilizer of halogenated organic solvents, rocket and racing fuel and a chemical intermediate. It is also used as a solvent for cyanoacrylate adhesives, polymers and waxes. It serves as a Michael donor, adding to alfa,beta-unsaturated carbonyl compounds through 1,4-addition in the Michael reaction. It acts as a solvent used for extractions, reaction medium and as a cleaning solvent. Further, it is used in the manufacture of pharmaceuticals, explosives, fibers and coatings.
Physical properties
Colorless liquid with a strong, disagreeable odor. Odor threshold concentration is 3.5 ppm (quoted, Amoore and Hautala, 1983).
Uses
Solvent; chemical synthesis; fuel for professional and model racing cars; in explosive mixtures
Uses
Most of the nitromethane produced in the United States (85% to 90%) is used in the synthesis of nitromethane derivatives used as pharmaceuticals, agricultural soil fumigants, and industrial antimicrobials (Markofsky 1991, Angus 2001). Nitromethane also is used as a fuel or fuel additive with methanol in racing cars, boats, and model engines. It formerly was used in the explosives industry as a component in a binary explosive formulation with ammonium nitrate and in shaped charges, and it was used as a chemical stabilizer to prevent decomposition of various halogenated hydrocarbons (NTP 1997, IARC 2000, Angus 2001).
Uses
Rocket fuel; solvent for zein. Used in the coating industry.
Definition
ChEBI: Nitromethane is a primary nitroalkane that is methane in which one of the hydrogens is replace by a nitro group. A polar solvent (b.p. 101 ℃), it is an important starting material in organic synthesis. It is also used as a fuel for rockets and radio-controlled models. It has a role as an EC 4.3.1.3 (histidine ammonia-lyase) inhibitor, a polar aprotic solvent and an explosive. It is a primary nitroalkane and a volatile organic compound.
Production Methods
Nitromethane and the other important nitroparaffins are synthesized commercially by the vapor-phase nitration of propane (Baker and Bollmeier 1978). At temperatures of 370-450°C and pressures of 8-12 atmospheres, nitromethane, nitroethane and 1- and 2-nitropropane are formed and then separated by distillation.
General Description
A colorless oily liquid. Flash point 95°F. May violently decompose if intensely heated when contaminated. Denser than water and slightly soluble in water. Hence sinks in water. Vapors are heavier than air. Moderately toxic. Produces toxic oxides of nitrogen during combustion.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Nitromethane may explode if heated or strongly shocked, especially if mixed with acids, bases [Handling Chemicals Safely 1980. p.687], acetone, aluminum powder, ammonium salts in the presence of organic solvents, haloforms (chloroform, bromoform), or hydrazine in methanol. Ignites on contact with alkyl aluminum or alkyl zinc halides. Reacts violently with strong bases (potassium hydroxide, calcium hydroxide), amines (1,2-diaminoethane, hydrazine), bromine, carbon disulfide, hydrocarbons, formaldehyde, metal oxides, lithium aluminum hydride, sodium hydride, strong oxidizing agents (lithium perchlorate, nitric acid, calcium hypochlorite). Reacts with aqueous silver nitrate to form explosive silver fulminate [Bretherick, 5th ed., 1995, p. 183]. Mixtures of Nitromethane and aluminum chloride may explode when organic matter is present [Chem. Eng. News 26:2257. 1948]. Nitromethane, either alone or in a mixture with methanol and castor oil, has a delayed but violent reaction with powdered calcium hypochlorite [Haz. Home Chem 1963]. Nitromethane reacts violently with hexamethylbenzene [Lewis 2544]. Nitromethane is strongly sensitized by hydrazine [Forshey, D. RR. et al, Explosivestoffe, 1969, 17(6), 125-129].
Hazard
Dangerous fire and explosion risk, lower explosion limit 7.3% in air. Toxic by ingestion and inhalation. Thyroid effects, upper respiratory tract irritant, and lung damage. Possible carcinogen.
Health Hazard
Nitromethane is used primarily as a chemical intermediate in the synthesis of biocides, chemicals, and agricultural products and intermediates. It is slightly toxic to aquatic organisms, has a low bioconcentration potential, and is considered not readily biodegradable. Acute toxicity is low following oral or dermal exposure. Nitromethane is a mild eye irritant and is not likely to cause significant irritation to the skin. Long-term excessive exposure may cause central nervous system effects. Based on animal data, nitromethane is classified as a Category 2B carcinogen (potential human carcinogen).
Health Hazard
Nitromethane is mildly irritating to the skin and mucous membranes (Gosselin et al 1976). It produces narcosis, mucus membrane irritation and central nervous system excitation, and some liver damage. These effects are generally not as marked as after administration of nitroethane. One case of human poisoning has been reported (Kaiffer et al 1972). In that case, a handyman was exposed to high concentrations of nitrocellulose and nitromethane resulting in a 67% conversion of his hemoglobin to methemoglobin and sulfhemoglobin. Treatment with hyperbaric oxygen, transfusion, peritoneal dialysis and then 6 sessions of hemodialysis resulted in recovery.
Fire Hazard
Behavior in Fire: Containers may explode
Industrial uses
Nitromethane is used as an intermediate in chemical syntheses, but more importantly it is used as a solvent for coatings and inks. It and the other nitroparaffins are excellent solvents for vinyls, epoxies, polyamides and acrylic polymers (Baker and Bollmeier 1978). It also is used as a military propellant and a racing fuel additive (HSDB 1988). Mixed with methanol and castor oil it is employed as a model airplane fuel.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by intravenous route. Mildly toxic by inhalation. In humans it may cause anorexia, nausea, vomiting, darrhea, kidney injury, and liver damage. exposed to heat, oxidizers, or flame. May explode by detonation, heat, or shock. Its sensitivity is increased when mixed with acids, bases, acetone, aluminum powder, ammonium salts + organic solvents, bis(2- aminoethyl)amine, 1,2-daminoethane + N,2,4,6-tetranitro-N-methyl aniLtne, halo forms (e.g., chloroform, bromoform), hydrazine + methanol. Ignites when mixed with alkyl metal halides (e.g., diethylaluminum bromide, dimethylaluminum bromide, ethylaluminum bromide iodide, methyl zinc iodide, methylaluminum diiodide). Can react violently with AlCl3 + organic matter, Ca(OH)2, m-methyl aniline, Ca(OCl)2, hexamethylbenzene, hydrocarbons, inorganic bases, hydroxides, organic amines, KOH, formaldehyde, nitric acid, metal oxides, 1,2-diaminomethane, litlvum perchlorate, sodium hydride. Reacts with aqueous silver nitrate to form the explosive silver fuhnate. When heated to decomposition it emits toxic fumes of NOx. See also NITROALKANES. A very dangerous fire hazard when exposed to heat, oxidizers, or flame. May explode by detonation, heat, or shock. Its sensitivity is increased when mixed with acids, bases, acetone, aluminum powder, ammonium salts + organic solvents, bis(2- aminoethyl)amine, 1,2-daminoethane + N,2,4,6-tetranitro-N-methyl aniLtne, halo forms (e.g., chloroform, bromoform), hydrazine + methanol. Ignites when mixed with alkyl metal halides (e.g., diethylaluminum bromide, dimethylaluminum bromide, ethylaluminum bromide iodide, methyl zinc iodide, methylaluminum diiodide). Can react violently with AlCl3 + organic matter, Ca(OH)2, m-methyl aniline, Ca(OCl)2, hexamethylbenzene, hydrocarbons, inorganic bases, hydroxides, organic amines, KOH, formaldehyde, nitric acid, metal oxides, 1,2-diaminomethane, litlvum perchlorate, sodium hydride. Reacts with aqueous silver nitrate to form the explosive silver fuhnate. When heated to decomposition it emits toxic fumes of NOx. See also NITROALKANES. concentrated sulfuric acid. When heated to decomposition it emits toxic fumes of NOx. See also NITRO COMPOUNDS and AMINES.
Potential Exposure
Nitromethane is used in the production of the fumigant, chloropicrin. It is best known as racing car fuel. It is also used as a solvent and as an intermediate in the pharmaceutical industry.
Carcinogenicity
Nitromethane is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Chemical/Physical. Nitromethane will not hydrolyze because it does not contain a hydrolyzable functional group.
Metabolism
Nitromethane is converted to nitrite and formaldehyde in a 1:1 ratio by hepatic microsomes from phenobarbital-pretreated male Sprague-Dawley rats (Sakurai et al 1980), but no formaldehyde could be detected when microsomes from the nose or liver of untreated male Fischer-344 rats were incubated with nitromethane (Dahl and Hadley 1983). Whether a similar conversion occurs in vivo has not been determined, but the absence of nitromethane metabolism in microsomes from untreated rats suggests that its metabolism in vivo may be slow.
Shipping
UN1261 Nitromethane, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Nitromethane is generally manufactured by gas-phase nitration of methane. The usual impurities include aldehydes, nitroethane, water and small amounts of alcohols. Most of these can be removed by drying with CaCl2 or by distillation to remove the water/nitromethane azeotrope, followed by drying with CaSO4. Phosphorus pentoxide is not suitable as a drying agent. [Wright et al. J Chem Soc 199 1936.] The purified material should be stored by dark bottles, away from strong light, in a cool place. Purifications using extraction are commonly used. For example, Van Looy and Hammett [J Am Chem Soc 81 3872 1959] mixed about 150mL of conc H2SO4 with 1L of nitromethane and allowed it to stand for 1 or 2days. The solvent was washed with water, aqueous Na2CO3, and again with water, then dried for several days with MgSO4, filtered again with CaSO4. It was fractionally distilled before use. Smith, Fainberg and Winstein [J Am Chem Soc 83 618 1961] washed it successively with aqueous NaHCO3, aqueous NaHSO3, water, 5% H2SO4, water and dilute NaHCO3. The solvent was dried with CaSO4, then percolated through a column of Linde type 4A molecular sieves, followed by distillation from some of this material (in powdered form). Buffagni and Dunn [J Chem Soc 5105 1961] refluxed it for 24hours with activated charcoal while bubbling a stream of nitrogen through the liquid. The suspension was filtered, dried (Na2SO4) and distilled, then passed through an alumina column and redistilled. It has also been refluxed over CaH2, distilled and kept under argon over 4A molecular sieves. It has been purified by zone melting at low temperature, or by distillation under vacuum at 0o, subjecting the middle fraction to several freeze-pump-thaw cycles. An impure sample containing higher nitroalkanes and traces of cyanoalkanes was purified (on the basis of its NMR spectrum) by crystallisation from diethyl ether at -60o (cooling in Dry-ice)[Parrett & Sun J Chem Educ 54 448 1977]. Fractional crystallisation is more effective than fractional distillation from Drierite in purifying nitromethane for conductivity measurements. [Coetzee & Cunningham J Am Chem Soc 87 2529 1965.] Specific conductivities around 5 x 10-9 ohm-1cm-1 were obtained. [Beilstein 1 IV 100.]
Toxicity evaluation
Nitromethane affects the central nervous system (CNS) via narcosis as a solvent. It is also a mild pulmonary irritant. In addition, nitromethane produces histidinemia in rats by decreasing hepatic histidase activity, leading to increased tissue levels of histidine.
Incompatibilities
May explode from heat, shock, friction, or concussion. Reacts with alkalis, strong acids; metallic oxides. Detonates or reacts violently with strong oxidizers, strong reducing agents such as hydrides; formaldehyde, copper, copper alloys; lead, lead alloys; hydrocarbons and other combustibles, causing fire and explosion hazard. Forms shock sensitive mixture when contaminated with acids, amines, bases, metal oxides; hydrocarbons, and other combustible materials.
Waste Disposal
Incineration: large quantities of material may require nitrogen oxide removal by catalytic or scrubbing processes.
Nitromethane Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
75-52-5(Nitromethane)Related Search:
1of4