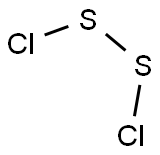
Disulfur dichloride
- CAS No.
- 10025-67-9
- Chemical Name:
- Disulfur dichloride
- Synonyms
- S2Cl2;SULFUR MONOCHLORIDE;SULFUR CHLORIDE;SULPHUR MONOCHLORIDE;ClSSCl;U.N. 1828;Chlorschwefel;Chlorosulfane;siarkichlorek;Sulfor choride
- CBNumber:
- CB2776712
- Molecular Formula:
- Cl2S2
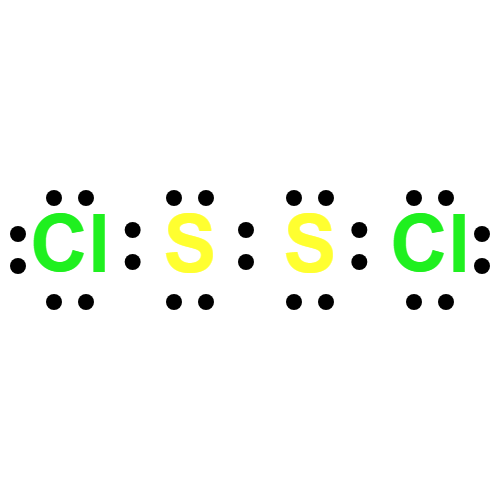
Lewis structure
- Molecular Weight:
- 135.04
- MDL Number:
- MFCD00011446
- MOL File:
- 10025-67-9.mol
- MSDS File:
- SDS
| Melting point | −80 °C(lit.) |
|---|---|
| Boiling point | 138 °C(lit.) |
| Density | 1.688 g/mL at 25 °C(lit.) |
| vapor density | 4.7 (vs air) |
| vapor pressure | 6.8 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | >130℃ |
| storage temp. | Store below +30°C. |
| solubility | soluble in No data available |
| form | yellow-red oily liquid |
| color | yellow-red oily liquid |
| explosive limit | 4.2-32.5%(V) |
| Water Solubility | reacts |
| Merck | 13,9060 |
| Dielectric constant | 4.8(15℃) |
| CAS DataBase Reference | 10025-67-9(CAS DataBase Reference) |
| FDA UNII | NJ7YR2EV0D |
| NIST Chemistry Reference | Disulfur dichloride(10025-67-9) |
| EPA Substance Registry System | Sulfur monochloride (10025-67-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H314-H400 | |||||||||
| Precautionary statements | P261-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T,C,N | |||||||||
| Risk Statements | 14-20-25-29-35-50-67-40-37-22 | |||||||||
| Safety Statements | 26-36/37/39-45-61 | |||||||||
| RIDADR | UN 3390 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WS4300000 | |||||||||
| Autoignition Temperature | 451 °F | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28121091 | |||||||||
| Toxicity | LD50 orally in Rabbit: 132 mg/kg | |||||||||
| IDLA | 5 ppm | |||||||||
| NFPA 704 |
|
Disulfur dichloride price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.14179 | Disulfur dichloride for synthesis | 10025-67-9 | 100ML | $64.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 157759 | Sulfur monochloride 98% | 10025-67-9 | 50g | $46.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.14179 | Disulfur dichloride for synthesis | 10025-67-9 | 1L | $315 | 2024-03-01 | Buy |
| Sigma-Aldrich | 157759 | Sulfur monochloride 98% | 10025-67-9 | 1kg | $122 | 2024-03-01 | Buy |
| Sigma-Aldrich | 293113 | Sulfur monochloride solution 1.0?M in methylene chloride | 10025-67-9 | 100ML | $50.1 | 2023-06-20 | Buy |
Disulfur dichloride Chemical Properties,Uses,Production
Physical Properties
Yellowish red oily liquid; pungent penetrating odor; fumes in air; refractive index 1.670 at 20°C; dipole moment 1.60; dielectric constant 4.9 at 22°C; reacts with water; soluble in ethanol, benzene, ether, chloroform, and carbon tetrachloride: dissolves sulfur at ambient temperature (67 g/100 g sulfur chloride).
Uses
Sulfur chloride is a solvent for sulfur and a chlorinating agent. Other applications are vulcanizing rubber; an intermediate in making sulfur dyes, synthetic rubber, thionyl chloride, and several other compounds; pesticide formulations; hardening soft woods; and extracting gold.
Preparation
Sulfur chloride is prepared as an orange liquid by passing chlorine gas through molten sulfur. If excess chlorine is passed and in the presence of a trace FeCl3 or iodine as catalyst, an equilibrium mixture of about 85% dichloride, SCl2 , and 15% S2Cl2 is obtained. The dichloride dissociates to sulfur chloride:
2SCl2 ↔ S2Cl2 + Cl2
Sulfur chloride is separated by fractional distillation.
Toxicity
Sulfur chloride is toxic and a lachrymator. The vapors can cause irritation of the eyes, nose, and respiratory tract.
Chemical Properties
Disulfur dichloride is a fuming, oily liquid with a yellowish-red to amber color and a suffocating odor. Soluble in alcohol, ether, benzene, carbon disulfide, and amyl acetate; decomposes on contact withwater. Combustible. It has an added hazard since it oxidizes and hydrolyzes to sulfur dioxide and hydrogen chloride.
Uses
Disulfur dichloride is used as Intermediate and chlorinating agent in the manufacture of organic chemicals, sulfur dyes, insecticides, synthetic rubbers; in cold vulcanization of rubber; as polymerization catalyst for vegetable oils; for hardening soft woods. The chemical fiber industry is used as a finishing agent for the manufacture of fabrics. The metallurgical industry is used as an extraction agent for precious and rare metals such as gold and silver.
Definition
Disulfur dichloride is a red fuming liquid with a strong smell. It is prepared by passing chlorine over molten sulfur and is used to harden rubber.
General Description
Sulfur monochloride appears as a yellow-red, oily, fuming liquid with a sharp odor. Contact or ingestion causes irritation or chemical burns to skin, eyes, and mucous membranes. Also poisonous by inhalation of vapors.
Health Hazard
Sulfur monochloride is an irritant of the eyes, mucous membranes, and skin. On contact with water, it decomposes to form hydrogen chloride and sulfur dioxide; because this occurs rapidly, it acts primarily as an upper respiratory irritant and does not ordinarily reach the lungs. However, exposure to high concentrations may cause pulmonary edema.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion and inhalation. A fuming, corrosive liquid very irritating to shin, eyes, and mucous membranes. It decomposes on contact with water to form the highly irritating hydrogen chloride, thiosulfuric acid, and sulfur. Its toxic effects are irritating to the upper respiratory tract, although the results of intoxication are usually transitory in nature. However, if hydrolysis is not complete in the upper respiratory tract, injury to the broncholes and alveoli can result. A fire hazard when in contact with organic matter, P203, Na2O2, water, Cr(OCl)2. Combustible when exposed to heat or flame. Will react with water or steam to produce heat and toxic and corrosive fumes. Can react with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of Cland SOX.
Potential Exposure
Sulfur chloride finds use as a chlorinating agent, catalyst, and as an intermediate in the manufacture of organic chemicals; carbon tetrachloride; sulfur dyes; insecticides, synthetic rubber; and pharmaceuticals. Exposure may also occur during the extraction of gold, purification of sugar juice; finishing and dyeing textiles; processing vegetable oils; hardening wood; and vulcanization of rubber. Has been used as a military poison.
Shipping
UN1828 Sulfur chlorides, Hazard class: 8; Labels: 8-Corrosive material, Potential Inhalation Hazard (Special Provision 5)
Purification Methods
It isa pungent, irritating golden yellow liquid. When impure its colour is orange to red due to SCl2 formed. It fumes in moist air and liberates HCl, SO2 and H2S in the presence of H2O. Distil it and collect the fraction boiling above 137o at atmospheric pressure. Fractionate this fraction over sulfur at ca 12mm using a ground glass apparatus (b 29-30o). Alternatively purify it by distillation below 60o from a mixture containing sulfur (2%) and activated charcoal (1%), under reduced pressure (e.g. 50mm). It is soluble in EtOH, *C6H6, Et2O, CS2 and CCl4. Store it in a closed container in the dark in a refrigerator. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 371 1963.] HARMFUL VAPOURS.
Incompatibilities
Decomposes violently in water, forming hydrochloric acid, sulfur dioxide; sulfur, sulfite, thiosulfate, and hydrogen sulfide. Reacts with oxidizers, strong bases; peroxides, phosphorus oxides; organics, antimony, antimony sulfide; arsenic sulfide; mercury oxide; tin, alkenes, terpenes, unsaturated glycerides; chromyl chloride; methyl sulfoxide; dimethylformamide, acetone, and other compounds; causing fire and explosion hazard. Corrosive to many metals in presence of water. Attacks some plastics, rubber and coatings.
Waste Disposal
Wearing protective equipment, spray carefully onto sodium ash/slaked lime mixture. Then spray with water, dilute, neutralize and flush to drain.
Precautions
Sulfur monochloride is minimally corrosive to carbon steel and iron when dry. When wet, it behaves like hydrochloric acid and attacks steel, cast iron, aluminum, stainless steels, copper and copper alloys, and many nickel-based materials.
Disulfur dichloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ARCTIC EXPORTS INC | +1-3026880818 +1-3026880818 | ARCTICEXPORTSINC@GMAIL.COM | Canada | 68 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
View Lastest Price from Disulfur dichloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-02-13 | Disulfur Dichloride
10025-67-9
|
US $1.00 / kg | 100kg | 99% | 1000000 | ARCTIC EXPORTS INC | |
 |
2019-07-06 | Disulfur dichloride
10025-67-9
|
US $10.00 / KG/Tin | 1KG | 99% | 100 | Career Henan Chemical Co |
-

- Disulfur Dichloride
10025-67-9
- US $1.00 / kg
- 99%
- ARCTIC EXPORTS INC
-

- Disulfur dichloride
10025-67-9
- US $10.00 / KG/Tin
- 99%
- Career Henan Chemical Co