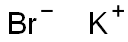Potassium bromide
- CAS No.
- 7758-02-3
- Chemical Name:
- Potassium bromide
- Synonyms
- KBr;BROMINE WATER;BROMINE TS;Kaliumbromid;Battery Grade;BROMINE LIQUID;Potassium bromide (KBr);ACS, 99% min;KALII BROMIDUM;BROMIDE BROMATE
- CBNumber:
- CB2854302
- Molecular Formula:
- BrK
- Molecular Weight:
- 119
- MDL Number:
- MFCD00011358
- MOL File:
- 7758-02-3.mol
- MSDS File:
- SDS
| Melting point | 734 °C (lit.) |
|---|---|
| Boiling point | 1435 °C/1 atm (lit.) |
| Density | 3.119 g/mL at 25 °C(lit.) |
| vapor density | 7.14 (vs air) |
| vapor pressure | 175 mm Hg ( 20 °C) |
| refractive index | 1.559 |
| Flash point | 1435°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | random crystals |
| Specific Gravity | 2.75 |
| color | White |
| PH | 5.0-8.8 (25℃, 50mg/mL in H2O) |
| Water Solubility | 650 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax |
λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,7618 |
| Dielectric constant | 4.8700000000000001 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids, bromine trifluoride and bromine trichloride. |
| InChIKey | IOLCXVTUBQKXJR-UHFFFAOYSA-M |
| LogP | 1 at 25℃ |
| Substances Added to Food (formerly EAFUS) | POTASSIUM BROMIDE |
| FDA 21 CFR | 173.315 |
| CAS DataBase Reference | 7758-02-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | OSD78555ZM |
| NIST Chemistry Reference | Potassium bromide(7758-02-3) |
| EPA Substance Registry System | Potassium bromide (7758-02-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38-36 | |||||||||
| Safety Statements | 26-45-61-7/9-39-36 | |||||||||
| RIDADR | UN 1744 8/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | TS7650000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28275100 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 2000 mg/kg | |||||||||
| NFPA 704 |
|
Potassium bromide price More Price(119)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P9881 | Potassium bromide ReagentPlus?, ≥99.0% | 7758-02-3 | 500G | $92.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | P9881 | Potassium bromide ReagentPlus?, ≥99.0% | 7758-02-3 | 1KG | $136 | 2024-03-01 | Buy |
| Sigma-Aldrich | 221864 | Potassium bromide FT-IR grade, ≥99% trace metals basis | 7758-02-3 | 25g | $170 | 2024-03-01 | Buy |
| Sigma-Aldrich | 221864 | Potassium bromide FT-IR grade, ≥99% trace metals basis | 7758-02-3 | 100g | $398 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04907 | Potassium bromide for IR spectroscopy Uvasol? | 7758-02-3 | 100g | $464 | 2024-03-01 | Buy |
Potassium bromide Chemical Properties,Uses,Production
Chemical Properties
Potassium bromide is a white or colourless crystalline solid with a pungent strong bitter and saline taste, slightly hygroscopic and soluble in water and very slightly soluble in ethanol and ether; cubic; r.d. 2.75; m.p. 734°C; b.p. 1435°C. Potassium bromide maybe prepared by the action of bromine on hot potassium hydroxide solutionor by the action of iron(III) bromideor hydrogen bromide on potassium carbonate solution. It is used widely in the photographic industry and is also used as a sedative. Because of itsrange of transparency to infrared radiation,KBr is used both as a matrix for solid samples and as a prism materialin infrared spectroscopy.
Uses
Potassium bromide was used as a secondary halide in combination with an iodide in the paper negative processes, the albumen on glass process, and the wet collodion processes. When silver bromide gelatin emulsion was invented, potassium bromide was the primary halide. It was also used in combination with either bichloride of mercury, copper sulfate, or potassium ferricyanide in photographic bleaches and as a restrainer in alkaline developers used for gelatin plates and developing-out papers.
Application
Potassium bromide is widely used in optics because KBr has a low refractive index and a wide spectral range into the infrared with nearly no absorption. As a result, KBr is widely used as infrared optical windows, as infrared beamsplitters, and as substrates for interferometers. Commonly KBr is used in transmission infrared spectroscopy as a media for powder samples. The KBr and powder are ground together and pressed, using a die, into a thin disc under vacuum. The disc suspends the sample without contributing to the transmitted signal. Potassium bromide has also been used in synthesis, commonly as a source of bromide ions. For example, double displacement of KBr and bismuth nitrate yielded nanosheets of bismuth oxybromide. Solutions of KBr have also been found to be useful shape-control agents or crystal-habit modifiers in formation of metal nanocrystals, including palladium nanorods and bimetallic platinum-paladium nanocrystals. KBr is a common source of bromide ions used as nucleophiles in organic chemistry.
Preparation
Potassium bromide was produced by the action of bromine on hot potassium hydroxide solution or Reacting elemental bromine with potassium hydroxide or potassium iodide will produce the potassium bromide salt:
KOH + Br2 → KBr + HOBr
KI + Br2 → KBr + I2
The reaction of bromine with potassium carbonate and urea is the basis of the process. The first step of the process involves the addition of K2SO4 to the potassium carbonate solution, followed by heating to 80 °C. After the lead-containing precipitate is removed by filtration, the bromine and urea are added, and the temperature and pH are adjusted to 30 °C and 6.0-6.5, respectively. Potassium bromide is recovered by recrystallization after reduction of volume of the reacting solution by evaporation. The sulfate can be removed from the solution by addition of BaBr2.
Definition
ChEBI: Potassium bromide is a metal bromide salt with a K(+) counterion. It is used in the manufacture of photographic film, developer, film thickener, toner and color photo bleach.
General Description
Potassium bromide is a white salt that crystallizes in the cubic rock salt structure, like sodium chloride. KBr is hygroscopic, deliquescent, highly soluble in water, and soluble in some polar organic solvents like glycerol, ethylene glycol, liquid ammonia, and hot ethanol, but insoluble in acetone. Aqueous solutions are neutral (pH about 7). When dissolved, KBr dissociates completely into its ions, making it a useful source of bromine ions in double displacement reactions or salt metathesis reactions. For example, this property was used in the production photographic films of silver bromide: KBr was reacted with silver nitrate to precipitate silver bromide, a salt that decomposes on exposure to light.
Air & Water Reactions
Water soluble.
Reactivity Profile
Potassium bromide is not in generally strongly reactive. A weak reducing agent, incompatible with oxidizing agents. Also incompatible with salts of mercury and silver. Violent reactions occur with bromine trifluoride. May react with nitrous ether spirit, many alkaloidal salts and starch. May also react with acids . Reacts with concentrated sulfuric acid to generate fumes of hydrogen bromide.
Hazard
Toxic by ingestion and inhalation
Fire Hazard
Flash point data for Potassium bromide are not available; however, Potassium bromide is probably nonflammable.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Potassium bromide (KBr) is used as an anticonvulsant and sedative. KBr is used for optical windows and prisms. KBr is transparent in the wide wavelength range from near ultraviolet to long wave infrared. It is employed in the sample preparation for infrared transmission spectra.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Large doses can cause central nervous system depression. Prolonged inhalation may cause skin eruptions. Mutation data reported. Violent reaction with BrF3. When heated to decomposition it emits toxic fumes of K2O and Br-. See also BROMIDES.
Veterinary Drugs and Treatments
Bromides are used both as primary therapy and as adjunctive therapy
to control seizures in dogs that are not adequately controlled by
phenobarbital (or primidone) alone (when steady state trough phenobarbital
levels are >30 mcg/mL for at least one month). While
historically bromides were only recommended for use alone in patients
suffering from phenobarbital (or primidone) hepatotoxicity,
they are more frequently used as a drug of first choice.
Although not frequently used, bromides are also considered
suitable by some for use in cats with chronic seizure disorders, but
cats may be more susceptible to the drug’s adverse effects.
Purification Methods
Crystallise the bromide from distilled water (1mL/g) between 100o and 0o. Wash it with 95% EtOH, followed by Et2O. Dry it in air, then heat it at 115o for 1hour, pulverize it, then heat it in a vacuum oven at 130o for 4hours. It has also been crystallised from aqueous30% EtOH, or EtOH, and dried over P2O5 under vacuum before heating in an oven.
Potassium bromide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 | admin@zhanyaobio.com | China | 2136 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12452 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Wuhan Ruichi Technology Co., Ltd | +8613545065237 | admin@whrchem.com | China | 164 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9353 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
Related articles
- The preparation method of potassium bromide
- Potassium bromide is an inorganic substance with a chemical formula of KBr and a relative molecular mass of 119.
- Apr 8,2022
View Lastest Price from Potassium bromide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-11 | Potassium Bromide (KBr)
7758-02-3
|
US $3500.00-2800.00 / ton | 500ton | 99.9 | 200tons | Shandong Juchuang Chemical Co., LTD | |
 |
2024-01-08 | Potassium bromide
7758-02-3
|
US $9.00 / KG | 1KG | 99% | 50000tons | Wuhan Boyuan Import & Export Co., LTD | |
 |
2024-01-08 | Potassium bromide
7758-02-3
|
US $9.00 / KG | 1KG | 99% | 50000tons | Wuhan Boyuan Import & Export Co., LTD |
-

- Potassium Bromide (KBr)
7758-02-3
- US $3500.00-2800.00 / ton
- 99.9
- Shandong Juchuang Chemical Co., LTD
-

- Potassium bromide
7758-02-3
- US $9.00 / KG
- 99%
- Wuhan Boyuan Import & Export Co., LTD
-

- Potassium bromide
7758-02-3
- US $9.00 / KG
- 99%
- Wuhan Boyuan Import & Export Co., LTD