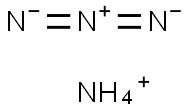ammonium azide
- CAS No.
- 12164-94-2
- Chemical Name:
- ammonium azide
- Synonyms
- ammonium azide
- CBNumber:
- CB2919935
- Molecular Formula:
- H4N4
- Molecular Weight:
- 60.05856
- MDL Number:
- MOL File:
- 12164-94-2.mol
ammonium azide Chemical Properties,Uses,Production
Chemical properties
Ammonium azide is an explosive chemical compound with the formula NH4N3.It is one of the few oxygen-free explosive materials that can be handled (relatively) safely.
Ammonium azide will detonate to release nitrogen and hydrogen gases. NH4N3 → 2 N2 + 2 H2
Traces of ammonia are also produced.
Physical properties
Ammonium azide is a non-hygroscopic colorless odorless crystalline solid. It melts at 160 °C and decomposes at 400 °C. Ammonium azide is soluble in water. It also is soluble in ammonia. It is also easily soluble in ethanol, glycerol, methanol, pyridine, sparingly soluble in allyl alcohol, butanol and isobutanol and insoluble in acetone, aniline, benzaldehyde, benzene, carbon disulfide, chlorobenzene, chloroform, diethyl ether, ethyl acetate, isoamyl alcohol, methyl acetate, methyl ethyl ketone, nitrobenzene, tetrachloroethane, toluene and xylene.
Preparation
Ammonium azide can be made by bubbling anhydrous ammonia in a ether solution of HN3. Since ammonium azide is almost insoluble in ether, it will precipitate.The precipitate is filtered and vacuum dried. Water traces are removed using phosphorus pentoxide in a desiccator.
Heating a mixture of sodium azide with ammonium nitrate in a stream of dry air in a tube at 190 °C for 30 min will yield ammonium azide. The yield of the process is 93%. Ammonium sulfate can also be used instead of nitrate.
http://www.sciencemadness.org
Chemical Properties
orthorhombic, a=0.893 nm, b=0.864 nm, c=0.380 nm [CIC73]
Safety Profile
Poison by inhalation and ingestion. See also AZIDES. Moderately flammable. Unstable. Explosion hazard upon rapid heating.