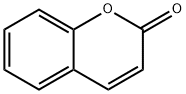
Coumarin
- CAS No.
- 91-64-5
- Chemical Name:
- Coumarin
- Synonyms
- 2H-Chromen-2-one;COUMARINE;2H-1-BENZOPYRAN-2-ONE;CUMARIN;CHROMEN-2-ONE;1,2-BENZOPYRONE;Kumarin;coumarin powder;1-BENZOPYRAN-2-ONE;Rattex
- CBNumber:
- CB3112168
- Molecular Formula:
- C9H6O2
- Molecular Weight:
- 146.14
- MDL Number:
- MFCD00006850
- MOL File:
- 91-64-5.mol
- MSDS File:
- SDS
| Melting point | 68-73 °C (lit.) |
|---|---|
| Boiling point | 298 °C (lit.) |
| Density | 0.935 |
| vapor pressure | 0.01 mm Hg ( 47 °C) |
| refractive index | 1.5100 (estimate) |
| Flash point | 162 °C |
| storage temp. | Store below +30°C. |
| solubility | 1.7g/l |
| form | Crystals or Crystalline Powder |
| color | White |
| PH Range | Non' uorescence (9.5) to light green ' uorescence (10.5) |
| Odor | at 10.00 % in dipropylene glycol. sweet hay tonka new mown hay |
| Odor Type | tonka |
| Water Solubility | 1.7 g/L (20 ºC) |
| λmax | 275nm |
| Merck | 14,2562 |
| BRN | 383644 |
| Major Application | color filter, organic electroluminescent devices, liquid crystal displays, field emission displays, inks, nickel plating, detergents, deodorant for shoes, petroleum products, cigarettes, personal care products, cosmetics, sunscreen cream, perfumes, nucleic acid sequencing, antiinflammatory agent, treatment of cancer, neurotransmission disorders, bleeding disorders, cerebrovascular disease, thrombosis, hemorrhoids, rheumatic disease, arthritic disease, epilepsy, vaginitis, painkiller, teeth whitening agent, skin whitening agent, wound healing promoter |
| InChIKey | ZYGHJZDHTFUPRJ-UHFFFAOYSA-N |
| LogP | 1.39 at 25℃ |
| FDA 21 CFR | 189.130 |
| Substances Added to Food (formerly EAFUS) | COUMARIN--PROHIBITED |
| CAS DataBase Reference | 91-64-5(CAS DataBase Reference) |
| EWG's Food Scores | 4-7 |
| FDA UNII | A4VZ22K1WT |
| NCI Dictionary of Cancer Terms | coumarin |
| NCI Drug Dictionary | coumarin |
| NIST Chemistry Reference | Coumarin(91-64-5) |
| IARC | 3 (Vol. Sup 7, 77) 2000 |
| EPA Substance Registry System | Coumarin (91-64-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311-H317 | |||||||||
| Precautionary statements | P261-P264-P270-P280-P301+P310-P302+P352+P312 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-40-36/37/38-20/21/22-43 | |||||||||
| Safety Statements | 36-36/37-26 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GN4200000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29322010 | |||||||||
| Toxicity | LD50 orally in rats, guinea pigs: 680, 202 mg/kg (Jenner) | |||||||||
| NFPA 704 |
|
Coumarin price More Price(60)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22316 | Coumarin for synthesis | 91-64-5 | 250g | $78.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22316 | Coumarin for synthesis | 91-64-5 | 1kg | $245 | 2024-03-01 | Buy |
| Sigma-Aldrich | 72609 | Coumarin certified reference material, TraceCERT | 91-64-5 | 100mg | $115 | 2024-03-01 | Buy |
| Sigma-Aldrich | 01260595 | Coumarin primary reference standard | 91-64-5 | 50mg | $308 | 2024-03-01 | Buy |
| Sigma-Aldrich | CRM40381 | Coumarin solution certified reference material, 2000?μg/mL in methanol, ampule of 1?mL | 91-64-5 | 1mL | $192 | 2022-05-15 | Buy |
Coumarin Chemical Properties,Uses,Production
Brief Introduction
It is also known as 1, 2-benzopyrone, cis ortho-caberillin, o-hydroxy cinnamon lactone and coumarin. It is contained in many natural plants in the form of glycosides and esters as vanillin instead of free-form. Coumarin will come out when certain plants are fermented and processed. Coumarin is found in the seeds of Dayton beans (Riccinechoides) in 1820 and is widely distributed in the plant kingdom, especially in plant species including Umbelliferae, Soybean, Rutaceae and Calyx. Seeds contain about 1.5% of the coumarin. In addition, coumarin is also contained in lavender oil, cinnamon oil and Peru balsam. Coumarin is spicy with sweet and lemongrass aroma. The aroma is emitted from the pink gum in the leaves of the fragrant beans, and the gum is made from the breakdown of the coumarin glycosides in the leaves. The aroma emitted by Sweet alfalfa is actually from the release of coumarin due to fermentation and decomposition during the stacking process. Precipitate from the ether appears as orthorhombic white pyramid or oblique sheet-like crystals with Lemongrass-type smell. It can subject to sublimation.
Chemical Properties
Golden crystalline solid (fronds or rhomboid); it is sweet with black beans-like aroma, dried herbs aroma and fennel aroma. After dilution, it smells like dried straw, nuts and tobacco. It is insoluble in cold water but soluble in hot water, ethanol and chloroform, easily soluble in ether and benzene. The solubility in 100ml of water at 25 ℃ is only 0.01g; 13 7g in 100ml of ethanol at 16 ℃; 1g in 50 mL 100℃ hot water. Oral LD50: 680mg / kg for rat.
Uses
used as a spice for the preparation of floral fragrances such as lavender, rosemary and rosemary, used in perfumes, cosmetics, soaps and detergents; used as flavoring agents for blending fragrances to make the aroma be lasting and unchanged; used as an electroplating additive to prevent the occurrence of pores in coating and can increase the brightness; as the flavor enhancer of printing ink and plastic; formerly used as spices and cigarettes spices, banned from 197; Since then, China had also prohibited it application in food; used as pharmaceutical raw materials.
Coumarin, as a laser dye, has an output laser range be within the blue-green region (420 ~ 570nm), has high fluorescence quantum efficiency, such as 7-ethylamino-6-methyl-4-trifluoromethyl coumarin Lactone 307), the structure is as follows: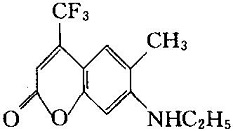
Description
Coumarin is a naturally occurring Benzopyrone compound. It is found in a large number of plants belonging to many different families including tonka beans, woodruff, lavender oil, cassia, melilot (sweet clover), and other plants. It is found in edible plants such as strawberries, cinnamon, peppermint, green tea, carrots, and celery, as well as in partially fermented tea, red wine, beer, and other foodstuffs. Concentrations range from 87 000 ppm in cassia and 40 000 ppm in cinnamon to 20 ppm in peppermint and 5 ppb in tangerines.
Chemical Properties
Coumarin occurs widely in nature and determines, for example, the odor of woodruff. It forms white crystals (mp 70.6°C) with a hay-like, spicy odor. When treated with dilute alkali, coumarin is hydrolyzed to the corresponding coumarinic acid salt [(Z)-2-hydroxycinnamic acid]. Heating with concentrated alkali or with sodium ethanolate in ethanol results in the formation of o-coumaric acid salts [(E)-2-hydroxycinnamic acid]. 3,4-Dihydrocoumarin is obtained by catalytic hydrogenation, for example, with Raney nickel as a catalyst; octahydrocoumarin is obtained if hydrogenation is carried out at high temperature (200–250°C).
Chemical Properties
WHITE CRYSTALS OR CRYSTALLINE POWDER
Chemical Properties
Coumarin has a sweet, fresh, hay-like, odor similar to vanilla seeds, and a burning taste with bitter undertone and nutlike flavor on dilution.
Occurrence
Found in many plants and essential oils such as cassia, melilot, orchid, lavender and balsam of Peru (Sp?th, 1937; Gildemeister & Hoffman, 1966).
Uses
antineoplastic, antiinflammatory, antihyperglycaemic
Uses
Pharmaceutic aid (flavor). Found in tonka beans, levender oil, woodruff, sweet clover.
Uses
coumarin is considered a blood thinner, it can also increase blood flow. Some sources cite anti-oxidant capacities, as well. It is a specific plant constituent and is what creates the fragrance of freshly mowed hay. Coumarin is found in such plants as cherries, lavender, licorice, and sweet clover.
Definition
ChEBI: A chromenone having the keto group located at the 2-position.
Preparation
Coumarin is currently produced by Perkin synthesis from salicylaldehyde.
In the presence of sodium acetate, salicylaldehyde reacts with acetic
anhydride to produce coumarin and acetic acid. The reaction is carried out in the
liquid phase at elevated temperature.
A process for the production of coumarin from hexahydrocoumarin by dehydrogenation has also been elaborated.
Since the odor of coumarin is relatively weak, strong-smelling by-products (e.g.,
vinylphenol) must be removed. Many purification methods have been reported
and patented.
Definition
A colorless crystalline compound with a pleasant odor, used in making perfumes. On hydrolysis with sodium hydroxide it forms coumarinic acid.
Aroma threshold values
Detection at 34 to 50 ppb; recognition, 250 ppb
Synthesis Reference(s)
The Journal of Organic Chemistry, 27, p. 4704, 1962 DOI: 10.1021/jo01059a541
Tetrahedron Letters, 27, p. 3911, 1986 DOI: 10.1016/S0040-4039(00)83914-3
General Description
Colorless crystals, flakes or colorless to white powder with a pleasant fragrant vanilla odor and a bitter aromatic burning taste.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Coumarin is sensitive to exposure to light. Coumarin is also sensitive to heat. Coumarin is incompatible with strong acids, strong bases and oxidizers. Coumarin is hydrolyzed by hot concentrated alkalis. Coumarin can be halogenated, nitrated and hydrogenated (in the presence of catalysts).
Hazard
Toxic by ingestion; carcinogenic. Use in food products prohibited (FDA). Questionable carcinogen.
Health Hazard
SYMPTOMS: Exposure to Coumarin may cause narcosis. It may also cause irritation and liver damage.
Fire Hazard
Coumarin is combustible.
Flammability and Explosibility
Non flammable
Biological Activity
Oral anticoagulants can be prepared from compounds with coumarin as a base. Coumarin has been known for well over a century and, in addition to its use pharmaceutically, it is also an excellent odor-enhancing agent. However, because of its toxicity, it is not permitted in food products in the United States (Food and Drug Administration). One commercial drug is 3-(alpha-acetonyl-4-nitrobenzyl)- 4-hydroxycoumarin. This drug reduces the concentration of prothrombin in the blood and increases the prothrombin time by inhibiting the formation of prothrombin in the liver. The drug also interferes with the production of factors VII, IX, and X, so that their concentration in the blood is lowered during therapy. The inhibition of prothrombin involves interference with the action of vitamin K, and it has been postulated that the drug competes with vitamin K for an enzyme essential for prothrombin synthesis. Another commercial drug is bis-hydroxy-coumarin, C19H12O6. The actions of this drug are similar to those just described.
Contact allergens
Coumarin is an aromatic lactone naturally occurring in Tonka beans and other plants. As a fragrance allergen, it has to be mentioned by name in cosmetics within the EU
Safety Profile
Poison by ingestion, intraperitoneal, and subcutaneous routes. Questionable carcinogen with experimental tumorigenic data. Experimental teratogenic effects. Mutation data reported. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and fumes. See also KETONES and ANHYDRIDES.
Synthesis
May be extracted from tonka beans; from salicylaldehyde and acetic anhydride in the presence of sodium acetate; also from o-cresol and carbonyl chloride followed by chlorination of the carbonate and fusion with a mixture of alkali acetate, acetic anhydride and a catalyst.
Environmental Fate
Coumarin toxicity is a function of blood and target tissue levels of coumarin relative to the metabolic capacity of the target organ. Cellular toxicity results when the formation of the toxic moieties exceeds the capacity of the cell to detoxify. This can have significant impact when comparing dosing by gavage to dietary exposure.
Purification Methods
Coumarin crystallises from ethanol or water and sublimes in vacuo at 43o [Srinivasan & deLevie J Phys Chem 91 2904 1987]. [Beilstein 17/10 V 143.]
Toxicity evaluation
Coumarin is readily biodegradable. Coumarin is unlikely to bind to soil. Coumarin does not bioaccumulate; the bioconcentration factor has been determined to be <10–40. Various environmental fate studies have shown that coumarin in the environment would biodegrade and be lost to volatilization. Losses resulting from photolysis may also occur.
Coumarin Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 1696 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3424 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2967 | 58 |
Related articles
- Uses of Coumarin
- Coumarin is a naturally occurring Benzopyrone compound. It is found in a large number of plants belonging to many different fa....
- Jan 12,2022
- Coumarin Metabolism, Toxicity and Carcinogenicity
- Coumarin is a natural product which exhibits marked species di?erences in both metabolism and toxicity. The majority of tests ....
- Nov 18,2019
- Coumarins — An Important Class of Phytochemicals
- Among them, coumarins are a family of benzopyrones (1,2-benzopyrones or 2H-1-benzopyran-2-ones) widely distributed in the natu....
- Nov 18,2019
View Lastest Price from Coumarin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Coumarin
91-64-5
|
US $0.00-0.00 / kg | 1kg | ≥98% HPLC | 1000kg | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-04-20 | Coumarin
91-64-5
|
US $3.00 / kg | 1kg | 99.92% | 50000tons | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2023-07-28 | Coumarin
91-64-5
|
US $0.00-0.00 / kg | 1kg | 0.99 | 50000kg | Hebei Yibangte Import and Export Co. , Ltd. |
91-64-5(Coumarin)Related Search:
1of4