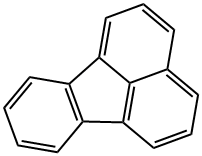
Fluoranthene
- CAS No.
- 206-44-0
- Chemical Name:
- Fluoranthene
- Synonyms
- Fluoranthen;Fluoranthrene;Fluoroanthene;1,2-Benzacenapthene;1,2-Benzacenaphthene;Idryl;FLUORANTHENE;fluoanthrene;Fluoranthene>Benzo[jk]flurene
- CBNumber:
- CB3123401
- Molecular Formula:
- C16H10
- Molecular Weight:
- 202.25
- MDL Number:
- MFCD00001184
- MOL File:
- 206-44-0.mol
- MSDS File:
- SDS
| Melting point | 105-110 °C (lit.) |
|---|---|
| Boiling point | 384 °C (lit.) |
| Density | 1.252 |
| refractive index | 1.0996 |
| Flash point | -18 °C |
| storage temp. | APPROX 4°C |
| solubility | Chloroform (Soluble), DMSO (Sparingly), Ethyl Acetate (Sparingly), Methanol (Spa |
| form | Crystalline Powder, Crystals and/or Chunks |
| color | Yellow or yellow-green to gray-beige |
| Water Solubility | insoluble |
| BRN | 1907918 |
| Henry's Law Constant | 5.53, 8.59, 13.0, 19.3, and 26.8(x 10-6 atm?m3/mol) at 4.1, 11.0, 18.0, 25.0, and 31.0 °C, respectively (Bamford et al., 1998) |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 206-44-0(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 360UOL779Z |
| NIST Chemistry Reference | Fluoranthene(206-44-0) |
| IARC | 3 (Vol. Sup 7, 92) 2010 |
| EPA Substance Registry System | Fluoranthene (206-44-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H410 | |||||||||
| Precautionary statements | P264-P270-P273-P301+P312-P391-P501 | |||||||||
| Hazard Codes | Xn,N,F,T | |||||||||
| Risk Statements | 22-36/37/38-40-67-65-50/53-38-11-39/23/24/25-23/24/25-52/53-50 | |||||||||
| Safety Statements | 37/39-26-36/37-24/25-23-62-61-60-45-16-7 | |||||||||
| RIDADR | UN 1593 6.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | LL4025000 | |||||||||
| HS Code | 29029090 | |||||||||
| Toxicity | LC50 (24-h) for Daphnia magna 1,300 mg/L (LeBlanc, 1980), Cyprinodon variegatus >560 ppm (H Acute oral LD50 for rats 2,000 mg/kg (quoted, RTECS, 1985). | |||||||||
| NFPA 704 |
|
Fluoranthene price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 48535 | Fluoranthene analytical standard | 206-44-0 | 5000mg | $62.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | BCR160R | Fluoranthene BCR?, certified reference material | 206-44-0 | 10MG | $296 | 2023-06-20 | Buy |
| Sigma-Aldrich | 11474 | Fluoranthene certified reference material, TraceCERT | 206-44-0 | 100mg | $112 | 2022-05-15 | Buy |
| TCI Chemical | F0016 | Fluoranthene >98.0%(GC) | 206-44-0 | 25g | $37 | 2024-03-01 | Buy |
| TCI Chemical | F0016 | Fluoranthene >98.0%(GC) | 206-44-0 | 100g | $98 | 2024-03-01 | Buy |
Fluoranthene Chemical Properties,Uses,Production
Description
Fluoranthene is a polycyclic hydrocarbon anda colorless crystalline solid. Molecular weight=202.26;Boiling point=about 375C; Freezing/Meltingpoint=111C. Hazard Identification (based on NFPA-704M Rating System): Health 0, Flammability 1, Reactivity 0.Virtually insoluble in water.
Chemical Properties
Fluoranthene is a polycyclic hydrocarbon and a colorless crystalline solid.
Uses
Fluoranthene can be used as a starting material in the synthesis of:
- Polyfluoranthene (PFA) based conducting polymer (PFA) by electrochemical anodic oxidation using Lewis acid catalyst.
- Substituted fluorenones.
- Fluorescence-emitting oligofluoranthene (OFA) nanorods by oxidative oligomerization.
Uses
Fluoranthene is a component of polynuclear aromatic hydrocarbons, also known as polycyclic aromatic hydrocarbons, and is usually bound to small particulate matter present in urban air, industrial and natural combustion emissions, and cigarette smoke.
Definition
ChEBI: An ortho- and peri-fused polycyclic arene consisting of a naphthalene and benzene unit connected by a five-membered ring.
Synthesis Reference(s)
Journal of the American Chemical Society, 72, p. 4786, 1950 DOI: 10.1021/ja01166a124
Tetrahedron Letters, 33, p. 1675, 1992 DOI: 10.1016/S0040-4039(00)91703-9
General Description
Light yellow fine crystals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as Fluoranthene, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.
Hazard
Questionable carcinogen.
Health Hazard
Fluoranthene exhibited mild oral and dermaltoxicity in animals. The acute toxicity is lowerthan that of phenanthrene. An oral LD50 valuein rats is reported as 2000 mg/kg. It may causeskin tumor at the site of application. However,any carcinogenic action from this compoundin animals is unknown..
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Fluoranthene emits acrid smoke and fumes.
Fire Hazard
Flash point data for Fluoranthene are not available. Fluoranthene is probably combustible.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion and skin contact. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Fluoranthene, a PAH, is produced from the pyrolytic processing of organic raw materials, such as coal and petroleum at high temperatures. It is also known to occur naturally as a product of plant biosynthesis. Fluoranthene is ubiquitous in the environment and has been detected in United States air; in foreign and domestic drink ing waters and in food-stuffs. It is also contained in ciga rette smoke. Individuals living in areas which are heavily industrialized; and in which large amounts of fossil fuels are burned, would be expected to have greatest exposure from ambient sources of fluoranthene. In addition, certain occupations e.g., coke oven workers, steelworkers, roofers, automobile mechanics) would also be expected to have elevated levels of exposure relative to the general popula tion. Exposure to fluoranthene will be considerably increased among tobacco smokers or those who are exposed to smokers in closed environments (i.e., indoors).
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Source
Detected in 8 diesel fuels at concentrations ranging from 0.060 to 13 mg/L with a mean
value of 0.113 mg/L (Westerholm and Li, 1994); in a distilled water-soluble fraction of used
motor oil at a concentration range of 1.3 to 1.5 μg/L (Chen et al., 1994). Lee et al. (1992) reported
concentration ranges 1.50-125 mg/L and ND-0.5 μg/L in diesel fuel and the corresponding
aqueous phase (distilled water), respectively (Lee et al., 1992). Schauer et al. (1999) reported
fluoranthene in a diesel-powered medium-duty truck exhaust at an emission rate of 53.0 μg/km.
Identified in Kuwait and South Louisiana crude oils at concentrations of 2.9 and 5.0 ppm,
respectively (Pancirov and Brown, 1975).
California Phase II reformulated gasoline contained fluoranthene at a concentration of 1.15
g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were approximately 4.25 and 160 μg/km, respectively (Schauer et al., 2002).
Detected in groundwater beneath a former coal gasification plant in Seattle, WA at a
concentration of 50 μg/L (ASTR, 1995). The concentration of fluoranthene in coal tar and the
maximum concentration reported in groundwater at a mid-Atlantic coal tar site were 6,500 and
0.015 mg/L, respectively (Mackay and Gschwend, 2001). Based on laboratory analysis of 7 coal
tar samples, fluoranthene concentrations ranged from 1,500 to 13,000 ppm (EPRI, 1990).Lehmann et al. (1984) reported fluoranthene concentrations of 64.7 mg/g in a commercial
anthracene oil and 17,400 to 30,900 mg/kg in three high-temperature coal tars. Identified in hightemperature
coal tar pitches used in roofing operations at concentrations ranging from 5,200 to
38,800 mg/kg (Arrendale and Rogers, 1981).
Fluoranthene was detected in soot generated from underventilated combustion of natural gas
doped with toluene (3 mole %) (Tolocka and Miller, 1995). Fluoranthene was also detected in 9
commercially available creosote samples at concentrations ranging from 55,000 to 120,000 mg/kg
(Kohler et al., 2000).
Detected in asphalt fumes at an average concentration of 20.48 ng/m3 (Wang et al., 2001).
An impurity in commercial available pyrene (Marciniak, 2002).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The respective gas-phase
and particle-phase emission rates of fluoranthene were 3.05 and 3.95 mg/kg of pine burned, 3.61
and 1.20 mg/kg of oak burned, and 3.75 and 0.509 mg/kg of eucalyptus burned.
Solubility in organics
In benzene expressed as mole fraction: 0.2174 at 44.8 °C, 0.3011 at 56.0 °C, 0.3826 at 64.4 °C,
0.5331 at 77.2 °C (shake flask-gravimetric, McLaughlin and Zainal, 1959)
In millimole fraction at 25 °C: 14.76 in n-hexane, 18.70 in n-heptane, 22.60 in n-octane, 26.42 in
n-nonane, 30.15 in n-decane, 50.46 in n-hexadecane, 18.07 in cyclohexane, 21.79 in methylcyclohexane,
30.11 in cyclooctane, 11.62 in 2,2,4-trimethylpentane, 24.82 in tert-butyl-cyclohexane,
51.77 in dibutyl ether, 47.55 in methyl tert-butyl ether, 2.67 in methanol, 5.44 in
ethanol, 6.70 in 1-propanol, 4.75 in 2-propanol, 9.96 in 1-butanol, 7.02 in 2-butanol, 4.95 in 2-
methyl-1-propanol, 14.46 in 1-pentanol, 19.86 1-hexanol, 25.24 in 1-heptanol, 31.25 in 1-
octanol, 10.21 in 2-pentanol, 8.62 in 3-methyl-1-butanol, 9.70 in 2-methyl-2-butanol, 17.72 in
cyclopentanol, 17.82 in 2-ethyl-1-hexanol, 11.72 in 2-methyl-1-pentanol, 0.948 in 4-methyl-2-
pentanol (Hernández and Acree, 1998)
storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from oxidizers. Where possible, automatically transfer material fromother storage containers to process containers.
Shipping
UN1325 Flammable solids, organic, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid.UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Fluoranthene (benzo[j,k]fluorene) M 202.3, m 110-111o, b 384o/760mm. Purify it by chromatography of CCl4 solutions on alumina, with *benzene as eluent. Crystallise it from EtOH, MeOH or *benzene. Also purify it by zone melting. [Gorman et al. J Am Chem Soc 107 4404 1985, Beilstein 5 I 344, 5 IV 2463.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Compound can react exo thermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel Crafts reaction.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must con form with EPA regulations governing storage, transportation, treatment, and waste disposal.
Fluoranthene Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
View Lastest Price from Fluoranthene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-03-22 | Fluoranthene
206-44-0
|
US $0.00-0.00 / KG | 1KG | 99% | 20 mt | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2021-07-13 | Fluoranthen
206-44-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-09 | Fluoranthen
206-44-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Fluoranthene
206-44-0
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Fluoranthen
206-44-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Fluoranthen
206-44-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd