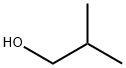
2-Methyl-1-propanol
- CAS No.
- 78-83-1
- Chemical Name:
- 2-Methyl-1-propanol
- Synonyms
- ISOBUTANOL;IBA;ISOBUTYL ALCOHOL;2-METHYLPROPAN-1-OL;i-Butanol;2-Methyl propanol;NATURAL ISO BUTYL ALCOHOL;1-propanol,2-methyl-;isobutano;2-methyl-1-propano
- CBNumber:
- CB3158955
- Molecular Formula:
- C4H10O
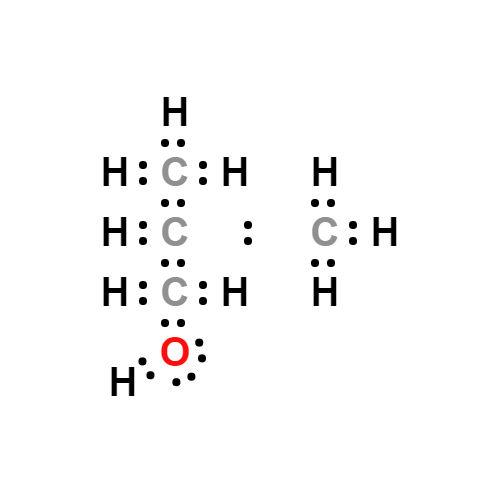
Lewis structure
- Molecular Weight:
- 74.12
- MDL Number:
- MFCD00004740
- MOL File:
- 78-83-1.mol
- MSDS File:
- SDS
| Melting point | ?108 °C (lit.) |
|---|---|
| Boiling point | 108 °C (lit.) 108 °C |
| Density | 0.803 g/mL at 25 °C (lit.) |
| vapor density | 2.55 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 2179 | ISOBUTYL ALCOHOL |
| Flash point | 82 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | water: miscible70g/L at 20°C |
| form | Solid |
| pka | >14 (Schwarzenbach et al., 1993) |
| color | APHA: ≤10 |
| Relative polarity | 0.552 |
| PH | 7 (80g/l, H2O, 20℃) |
| Odor | Slightly suffocating; nonresidual alcoholi |
| explosive limit | 1.5-12%(V) |
| Odor Threshold | 0.011ppm |
| Odor Type | ethereal |
| Water Solubility | 95 g/L (20 ºC) |
| λmax |
λ: 260 nm Amax: 0.10 λ: 280 nm Amax: 0.06 |
| JECFA Number | 251 |
| Merck | 14,5131 |
| BRN | 1730878 |
| Henry's Law Constant | 20.0 at 30.00 °C, 72.2 at 50.00 °C, 133 at 60.00 °C, 216 at 70.00 °C, 330 at 80.00 °C (headspace- GC, Hovorka et al., 2002) |
| Exposure limits | TWA 300 mg/m3 (100 ppm) NIOSH, 150 mg/m3 (50 ppm) (ACGIH); IDLH 8000 ppm. |
| Dielectric constant | 31.7(-80℃) |
| Stability | Stable. Flammable. Incompatible with strong oxidizing agents, aluminium. |
| InChIKey | ZXEKIIBDNHEJCQ-UHFFFAOYSA-N |
| LogP | 1 at 25℃ |
| Substances Added to Food (formerly EAFUS) | ISOBUTYL ALCOHOL |
| FDA 21 CFR | 172.515; 176.200; 177.2800; 73.1 |
| CAS DataBase Reference | 78-83-1(CAS DataBase Reference) |
| FDA UNII | 56F9Z98TEM |
| NIST Chemistry Reference | 1-Propanol, 2-methyl-(78-83-1) |
| EPA Substance Registry System | Isobutanol (78-83-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H315-H318-H335-H336 | |||||||||
| Precautionary statements | P210-P233-P240-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 10-37/38-41-67 | |||||||||
| Safety Statements | 13-26-37/39-46-7/9 | |||||||||
| RIDADR | UN 1212 3/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | NP9625000 | |||||||||
| Autoignition Temperature | 801 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29051990 | |||||||||
| Toxicity | LD50 orally in rats: 2.46 g/kg (Smyth) | |||||||||
| IDLA | 1,600 ppm | |||||||||
| NFPA 704 |
|
2-Methyl-1-propanol price More Price(56)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W217913 | Isobutyl alcohol natural, ≥99%, FCC, FG | 78-83-1 | 1kg | $81.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | W217913 | Isobutyl alcohol natural, ≥99%, FCC, FG | 78-83-1 | 8kg | $338 | 2024-03-01 | Buy |
| Sigma-Aldrich | W217905 | Isobutyl alcohol ≥99%, FCC, FG | 78-83-1 | 2Kg | $106 | 2024-03-01 | Buy |
| Sigma-Aldrich | W217905 | Isobutyl alcohol ≥99%, FCC, FG | 78-83-1 | 8kg | $180 | 2024-03-01 | Buy |
| Sigma-Aldrich | W217905 | Isobutyl alcohol ≥99%, FCC, FG | 78-83-1 | 20kg | $343 | 2024-03-01 | Buy |
2-Methyl-1-propanol Chemical Properties,Uses,Production
Description
Isobutanol, also known as isopropyl alcohol or 2-methyl propanol, is a colorless and flammable liquid. It is one of the main ingredients of fresh tea leaves, black tea and green tea to produce the wonderful aroma. The molecular weight of isobutanol is 74.12, with a boiling point of 107.66 ℃, a relative density of 0.8016, a refractive index of 1.3959, and a flash point of 37 ℃. It is soluble in alcohol and ether, but only slightly soluble in water. Isobutanol can form explosive mixtures with air, with an explosion limit of 2.4% (volume). It can also form addition compounds, such as CaCl2 • 3C4H10O, with calcium chloride.
Chemical properties
It is a kind of colorless transparent liquid with a special smell, soluble in water, and fully dissolved in ethanol and ether.
Uses
(1) For analysis reagents, chromatography reagents, solvents and extraction agent.
(2) As raw materials for the organic synthesis, and also act as a superior solvent.
(3) Isobutanol is raw materials for organic synthesis. It mainly used in the synthesis of isobutyronitrile, an intermediate for diazinon.
(4) As raw materials of organic synthesis, isobutanol is used in the manufacture of petroleum additives, antioxidants, 2, 6-butylated hydroxytoluene, isobutyl acetate (paint solvents), plasticizers, synthetic rubber, artificial musk, fruit oil and synthetic drugs. It can also be used to purify strontium, barium and lithium salts and other chemical reagents and used as a superior solvent.
(5) Extraction solvent. Food flavors listed in GB 2760-96.
Production method
(1) In the presence of sodium amalgam or other catalysts, it is derived from the reduction of butyraldehyde;r it is derived from the distillation of the by-product obtained from the synthesis of methanol.
(2) 1. Carbonyl synthesis (by-product of butanol production from propylene) : using propylene and synthesis gas as raw material, going through carbonyl synthesis in the system to get n-butyl and isobutyl aldehyde, after the catalyst, hydrogenating into alcohol, then undergoing dehydration and separation can obtain the product of n-butyl and isobutyl alcohol respectively.
2. Hydrogenation of isobutyraldehyde: isobutyraldehyde via the liquid hydrogenation reaction, obtained isobutanol in the catalytic nickel.
3. Recycle from isobutyl oil, a byproduct of synthetic methanol distillation: isobutanol is obtained by dehydration of methanol, salting out and then azeotropic distillation of isobutyl oil.
(3) The preparation method is using synthesis gas and propylene as raw materials, obtaining isobutyraldehyde during the carbonyl synthesis of 2-ethylhexyl alcohol, and then by hydrogenation to acquire isobutanol.
Hazards & Safety Information
Category: Flammable liquids
Toxic classification: Moderately hazardous
Acute toxicity: Oral-Rat LD50: 2460 mg/kg; Abdominal-Mouse LD50: 1801 mg/kg
Stimulation data: Eye-Rabbit 2 mg Severity
Explosives hazardous characteristics: To be explosive when mix with air.
Flammability hazard characteristics: Flammable in case of fire, high temperature, oxidant; stimulating the smoke when combustion.
Storage and transportation characteristics: Ventilation and low-temperature drying in treasury; oxidants and acids stored separately.
Extinguishing agent: Dry powder, dry sand, carbon dioxide, foam.
Occupational Standard: TWA 50 PPM.
Description
Isobutyl alcohol has a disagreeable odor. May be prepared from isobutylene; by reduction of isobutyraldehyde with sodium amalgam or in the presence of a catalyst; by fermentation of isobutyraldehyde; isolated during fermentation of carbohydrates.
Chemical Properties
Isobutyl alcohol has a penetrating, wine-like, disagreeable odor
Physical properties
Clear, colorless, oily liquid with a sweet, musty odor. Burning taste. The average least detectable odor threshold concentration in water at 60 °C was 0.36 mg/L (Alexander et al., 1982). Experimentally determined detection and recognition odor threshold concentrations were 2.0 mg/m3 (660 ppbv) and 5.4 mg/m3 (1.8 ppmv), respectively (Hellman and Small, 1974). An odor threshold concentration of 11 ppbv was reported by Nagata and Takeuchi (1990).
Occurrence
Reported found in the essential oils of Java citronella, tea, Eucalyptus amygdalina. Also reported in apple and currant aromas; in apricots, banana, sweet cherry, orange, grapefruit and tangerine juice, berries, guava, grapes, melon, papaya, pear, pineapple, leek, peas, rutabaga, tomato, ginger, spearmint oil, vinegar, breads, cheeses, milk, fish oil, meats, hop oil, beer, cognac, rum, whiskies, sherry, cider, grape wines, cocoa, tea, coffee, nuts, oats, soybean, avocado, olive, passion fruit, plum, beans, mango, starfruit, bantu beer, plum brandy, tamarind, fig, cardamom, gin, quince, radish, prickly pear, litchi, sukiyaki, lovage leaf, buckwheat, sweet corn, laurel, malt, wort, elderberry juice, dried bonito, krill, kiwifruit, loquat, fruit brandies and wines, endive, shrimp, truffle, red currants, Roman chamomile oil and other sources.
Uses
2-Methyl-1-propanol (Isobutyl alcohol) may be used to prepare the isobutyl alcohol-benzene solution for use in the quantification of inorganic phosphates. It may be used for the estimation of phosphate (inorganic phosphorous) by a colorimetric method.
Uses
Isobutanol is commonly used to produce isobutyl acetate for lacquers, isobutylphthalate for plasticizers, and as a solvent for plastics, textiles, oils, and perfumes. It is also used in the production of fruit flavoring esters and as a solvent in paint and varnish removers.
Uses
Isobutyl Alcohol is a reagent used in organic reactions. It is used in the synthesis of new fluorinating reagents. It is also used in the lipase-catalyzed production of biodiesel as an energy source.
Definition
ChEBI: Isobutanol is an alkyl alcohol that is propan-1-ol substituted by a methyl group at position 2. It has a role as a Saccharomyces cerevisiae metabolite. It is a primary alcohol and an alkyl alcohol. It derives from a hydride of an isobutane.
Preparation
From isobutylene; by reduction of isobutyraldehyde with sodium amalgam or in the presence of a catalyst; by fermentation of isobutyraldehyde; isolated during fermentation of carbohydrates
Production Methods
Isobutanol is commercially produced almost exclusively by the hydrogenation of isobutyraldehyde obtained from propylene using the oxo process.
Aroma threshold values
Detection: 360 ppb to 3.3 ppm
General Description
A clear colorless liquid with a sweet odor. Flash point 85 - 100°F. Less dense than water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
2-Methyl-1-propanol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. 2-Methyl-1-propanol is incompatible with strong oxidizers.
Hazard
Flammable, moderate fire risk. Strong irritant.
Health Hazard
Inhalation causes eye and throat irritation andheadache. Ingestion may cause depression ofthe central nervous system. It is an irritantto the skin, causing cracking. Target organsare the eyes, skin, and respiratory system.
LD50 value, oral (rabbits): 3750 mg/kg.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Flammable
Potential Exposure
Butyl alcohols are used as solvents for paints, lacquers, varnishes, natural and synthetic resins, gums, vegetable oils, dyes, camphor, and alkaloids. They are also used as an intermediate in the manufacture of pharmaceuticals and chemicals; in the manufacture of artificial leather, safety glass; rubber and plastic cements, shellac, raincoats, photographic films, perfumes; and in plastic fabrication.
Carcinogenicity
Nineteen Wistar rats were dosed with 0.2mL of isobutanol twice weekly by oral intubation. The average survival time was 495 days. It was reported that malignant tumors developed in three animals; one had a forestomach carcinoma and a liver cell carcinoma, another had a forestomach carcinoma and myelogenous leukemia, and the third, a myelogenous leukemia. In the same study, 24 rats were injected subcutaneously with 0.05 mL/kg twice weekly. The average survival time was 544 days. A total of eight malignant tumors developed: two forestomach carcinomas, two liver sarcomas, one spleen sarcoma, one mesothelioma, and two retroperitoneal sarcomas. Increased incidences of total tumors were observed by both routes of administration, but there was no significant increased incidence of any tumor type at any site. This study is considered inappropriate for cancer risk assessment.
Source
A product of whiskey fermentation (quoted, Verschueren, 1983). Isobutyl alcohol also occurs in tea leaves and java cintronella plants (Duke, 1992).
Shipping
UN1120 Butanols, Hazard Class: 3; Labels: 3— Flammable liquid. UN1212 Isobutanol or Isobutyl alcohol, Hazard Class: 3; Labels: 3—Flammable liquid
Purification Methods
Isobutanol is dried by refluxing with CaO and BaO for several hours, followed by treatment with calcium or aluminium amalgam, then fractional distilling it from sulfanilic or tartaric acids. More exhaustive purifications involve formation of phthalate or borate esters. Heating it with phthalic anhydride gives the acid phthalate which, after crystallisation to constant melting point (m 65o) from pet ether, is hydrolysed with aqueous 15% KOH. The alcohol is distilled off as the water azeotrope and dried (K2CO3, then anhydrous CuSO4), and finally magnesium turnings, followed by fractional distillation. [Hückel & Ackermann J Prakt Chem 136 15 1933.] The borate ester is formed by heating the dried alcohol for 6hours in an autoclave at 160-175o with a quarter of its weight of boric acid. After fractional distillation under vacuum, the ester is hydrolysed by heating for a short time with aqueous alkali and the alcohol is dried with CaO and distilled. [Michael et al. J Am Chem Soc 38 653 1916.] Alternatively dry the alcohol with K2CO3, CaSO4 or CaCl2, filter and fractionally distil it. For further drying, the redistilled alcohol can be refluxed with the appropriate alkyl phthalate or succinate as described under ethanol. [Beilstein 1 IV 1588.]
Incompatibilities
Butyl alcohols may form explosive mixture with air. In all cases they are Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks some plastics, rubber and coatings. n-Butanol is incompatible with strong acids; halogens, caustics, alkali metals; aliphatic amines; isocyanates. sec-Butanol forms an explosive peroxide in air. Ignites with chromium trioxide. Incompatible with strong oxidizers; strong acids; aliphatic amines; isocyanates, organic peroxides. tert-Butanol is incompatible with strong acids (including mineral acid), including mineral acids; strong oxidizers or caustics, aliphatic amines; isocyanates, alkali metals (i.e., lithium, sodium, potassium, rubidium, cesium, francium). isoButanol is incompatible with strong acids; strong oxidizers; caustics, aliphatic amines; isocyanates, alkali metals and alkali earth. May react with aluminum at high temperatur
Waste Disposal
Incineration, or bury absorbed waste in an approved land fill.
2-Methyl-1-propanol Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shandong Quark Chemical Co., Ltd. | +8613325218432 | sales@quarkchem.com | China | 57 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
View Lastest Price from 2-Methyl-1-propanol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-11 | Isobutyl Alcohol
78-83-1
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-07-27 | 2-Methyl-1-propanol
78-83-1
|
US $1.50 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd | |
 |
2023-06-07 | 2-Methyl-1-propanol
78-83-1
|
US $10.00 / KG | 1KG | 99.% | 10 ton | Hebei Guanlang Biotechnology Co,.LTD |
-

- Isobutyl Alcohol
78-83-1
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- 2-Methyl-1-propanol
78-83-1
- US $1.50 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
-

- 2-Methyl-1-propanol
78-83-1
- US $10.00 / KG
- 99.%
- Hebei Guanlang Biotechnology Co,.LTD
78-83-1(2-Methyl-1-propanol)Related Search:
1of4