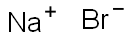Sodium bromide
- CAS No.
- 7647-15-6
- Chemical Name:
- Sodium bromide
- Synonyms
- NaBr;BROMIDE;Natriumbromid;Sodium bromide (NaBr);BROMIDE STANDARD;sodium bromide solution;Hydrobromic acid sodium salt;Sedoneural;bromnatrium;SODIUM BROMID
- CBNumber:
- CB3181001
- Molecular Formula:
- NaBr
- Molecular Weight:
- 102.89
- MDL Number:
- MFCD00003475
- MOL File:
- 7647-15-6.mol
- MSDS File:
- SDS
| Melting point | 755 °C (lit.) |
|---|---|
| Boiling point | 1390 °C |
| Density | 3,203 g/cm3 |
| vapor pressure | 1 mm Hg ( 806 °C) |
| refractive index | 1.6412 |
| Flash point | 1390°C |
| storage temp. | Store at room temperature. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Powder |
| color | White |
| Specific Gravity | 3.21 |
| PH | 5.74 (430g/l, H2O, 22.5℃) |
| Water Solubility | 905 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax |
λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,8594 |
| BRN | 3587179 |
| Dielectric constant | 6.3399999999999999 |
| Stability | Stable. Incompatible with strong acids. Hygroscopic. |
| InChIKey | JHJLBTNAGRQEKS-UHFFFAOYSA-M |
| LogP | 0 at 25℃ |
| Indirect Additives used in Food Contact Substances | SODIUM BROMIDE |
| CAS DataBase Reference | 7647-15-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | LC1V549NOM |
| NIST Chemistry Reference | Sodium bromide(7647-15-6) |
| Pesticides Freedom of Information Act (FOIA) | Sodium bromide |
| EPA Substance Registry System | Sodium bromide (7647-15-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P280-P302+P352-P362+P364 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 24/25-25-36-26-22 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | VZ3150000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28275100 | |||||||||
| Toxicity | LD50 orally in rats: 3.5 g/kg (Smith, Hambourger) | |||||||||
| NFPA 704 |
|
Sodium bromide price More Price(61)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SX0390 | Sodium Bromide Meets ACS Specifications GR ACS | 7647-15-6 | 500g | $107 | 2024-03-01 | Buy |
| Sigma-Aldrich | NIST977 | Isotopic standard for bromine NIST? SRM? 977 | 7647-15-6 | 0.25G | $731 | 2024-03-01 | Buy |
| Sigma-Aldrich | 220345 | Sodium bromide ReagentPlus , ≥99% | 7647-15-6 | 2.5kg | $267 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1613597 | Sodium bromide United States Pharmacopeia (USP) Reference Standard | 7647-15-6 | 1g | $381 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.19896 | Bromide standard solution traceable to SRM from NIST NaBr in H?O 1000 mg/l Br Certipur? | 7647-15-6 | 500mL | $55.1 | 2024-03-01 | Buy |
Sodium bromide Chemical Properties,Uses,Production
Physical and chemical properties
sodium bromide is a colorless cubic crystal or white granular powder, and belongs to isometric system. It is odorless, and has slightly bitter and briny taste but high toxicity. It is easily to absorb moisture and caking but without deliquescence. It is slightly soluble in alcohol and easily soluble in water (at 100 °C, the solubility in 100ml water solubility is 121g), its aqueous solution is neutral with electronic conductivity. The anhydrous sodium bromide crystal will be precipitated out at 51°C with dihydrate compound forming at temperature lower than 51 °C. Its bromide ion can be substituted by fluorine, and chlorine. Under acidic conditions, it can be oxidized by oxygen and release free bromine; this process is taken advantage of by industry for producing bromine. It can have reaction with dilute sulfuric acid to produce hydrogen bromide. However, hydrobromic acid is a strong acid which can’t be produced through the reaction with dilute sulfuric acid and can only made through high-boiling point acid to make low-boiling point acid. However, we should avoid to use concentrated sulfuric acid which has strong oxidation effect and thus converting bromine (-1) into bromine element and release reddish-brown gas. This method can be used to identify sodium iodide (Heating sodium iodide and concentrated sulfuric acid together will release red-purple gases), Thereby, we can only take the concentrated phosphoric acid together with sodium bromine for heating to produce hydrogen bromine.

Bromide ions can enhance the inhibitor process of brain cortex, and promote their concentration. Therefore, medically it can be used as tranquilizers, and hypnotic or anticonvulsant drugs. When human swallow or inhale the compounds, it will cause harm to central nervous system, brain, and eye while causing irritation response of skin, eyes and also the respiratory tract.
Chemical Properties
Sodium bromide is a colorless cubic crystal or white granular powder. It is odorless, and has slightly bitter and briny taste but high toxicity. It is easily soluble in water (at 100 °C, the solubility in 100ml water solubility is 121g), but slightly soluble in alcohol.
Uses
- Sodium bromide can be used as raw material in the preparation of liquid photographic film; medically as sedative, the brominating agent in printing and dyeing; it can also be used in synthetic fragrances and other chemicals.
- Photographic industry applies it for the preparation of liquid photosensitive film. It is medically used for the production of diuretics and sedatives. Perfume industry uses it for the production of synthetic fragrances. Printing and dyeing industry use it as a brominating agent. In addition, it can be also be used for organic synthesis and so on.
- Sodium bromide is used for the photographic industry, spices, pharmaceutical and printing industries.
- It is used for the reagents for analysis, and can also be used for the synthesis of inorganic and organic compounds and pharmaceutical industry.
- It is sued for photographic film, medicines, perfumes, dyes and other industries.
- It can be applied to determination of trace cadmium and Manufacturing of bromide. It can also be applied to inorganic and organic synthesis, photogravure and pharmaceuticals.
Production method
Urea reduction: dissolve soda ash (sodium carbonate), urea in hot water, and fed into the reactor; gradually add bromine for reaction and generate sodium bromide. Then further add active carbon for decolorization; further undergo filtration, evaporation, crystallization, centrifugal separation, and drying to obtain sodium bromide products. The reaction is as following:
3Br2 + 3Na2CO3 + NH2CONH2 → 6NaBr + 4CO2 ↑ + N2 ↑ + 2H2O
Neutralization method: add about 40% hydrobromic acid into the reactor, stir and slowly add 40% caustic solution for neutralization to Ph 7.5~8 for generating sodium bromide; after isolated by centrifugation, evaporation, crystallization and centrifuged again separation, then we can obtain the final product of sodium bromide. the reaction is:
HBr + NaOH → NaBr + H2O
Toxicity
We should prevent its ingestion and inhalation; avoid the contact of eye and skin with it. If intake or inhalation happens, adverse reactions include dizziness, nausea, and vomiting can occur. In these cases, we should immediately consult a doctor for treatment. Upon being splashed in the eyes, we should immediately rinse with fresh water for 20 min; upon skin contact with sodium bromide, we should also rinse with plenty of water.
Chemical Properties
Sodiumbromide, NaBr,is a white, hygroscopic, crystalline solid with a bitter, saline taste.It is water soluble,with a melting point of 758°C (1400 OF). Sodium bromide is used in medicine as a sedative and in photography in the preparation of silver bromide emulsion on photographic plates or films.
Physical properties
White crystalline powder or granules; saline and slight bitter taste; cubic structure; density 3.20 g/cm3; melts at 747° C; vaporizes at 1,390°C; vapor pressure 1 torr at 806°C and 5 torr at 903°; highly soluble in methanol, 16.7 g/100mL.
The dihydrate is a white crystalline solid; density 2.18 g/cm3; decomposes at 36°C; soluble in water; sparingly soluble in methanol.
Occurrence
Sodium bromide occurs in seawater at an average concentration of 0.008%. It also is found naturally in some salt deposits. It is used in photography for preparing light-sensitive silver bromide emulsions. The salt also is used as a bleaching and disinfecting agent for water treatement in swimming pools, health spas, and hot tubs. Other uses are as a catalyst for partial oxidation of hydrocarbons, for increasing density of aqueous drillng fluids for oil wells, as an electrolyte component in sodium-halogen batteries, as a brominating agent in organic synthesis, in preparing bromide salts, and as a laboratory reagent. Sodium bromide is used in medicine as a sedative and hypnotic.
Uses
Sodium Bromide is an inorganic compoiund used as a catalyst in the photoinduced polymerization of acrylates.
Uses
Sodium Bromide is a high-tonnage chemical and one of the most important of the bromide salts (NaBr2). High-purity grades are required in the formulation of silver bromide emulsions for photography. The compound, usually in combination with hypochlorites, is used as a bleach, notably for cellulosics. The production of sodium bromide simply involves the neutralization of HBr with NaOH or with sodium carbonate or bicarbonate.
Preparation
Sodium bromide can be prepared by several methods. Pure salt can be made by neutralizing sodium hydroxide or sodium carbonate with hydrobromic acid. The solution is evaporated for crystallization:NaOH + HBr → NaBr + H2O NaCO3 + HBr → NaBr + CO2 + H2O
Sodium bromide can be made by passing bromine through an aqueous solution of sodium hydroxide or carbonate in the presence of a reducing agent, such as ammonia, hydrazine, activated charcoal, or Fe2+ ion. A typical method involves adding iron to bromine water to form ferrosoferric bromide, Fe[FeBr5]. This double salt is dissolved in excess water followed by addition of sodium carbonate. The product mixture is filtered and the filtrate is evaporated to crystallize sodium bromide. The overall reaction may be written as follows: 3Fe + 4Br2 + 4Na2CO3 → 8NaBr + FeCO3 + Fe2(CO3)3
Another method involves adding excess bromine to a solution of sodium hydroxide. This forms sodium bromide and bromate. The product solution is evapoated to dryness. The bromate is reduced to bromide by heating with carbon: 3Br2 + 2NaOH + H2O → NaBr + NaBrO3 + 4HBr.
Definition
ChEBI: Sodium bromide is an inorganic sodium salt having bromide as the counterion. It is a bromide salt and an inorganic sodium salt. It is used in photographic processingand in analytical chemistry.
General Description
Sodium bromide is a brominating agent mainly used in organic synthetic reactions as a bromide source.
Hazard
Toxic by inhalation and ingestion.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Sodium bromide can be used as a substitute for potassium bromide.
Safety Profile
Moderately toxic by ingestion. Experimental reproductive effects. Incompatible with acids, alkaloidal and heavy-metal salts. When heated to decomposition it emits toxic fumes of Brand NazO. See also BROMIDES.
Purification Methods
Crystallise the bromide from water (0.86mL/g) between 50o and 0o, and dry it at 140o under vacuum (this purification may not eliminate chloride ion).
Incompatibilities
Sodium bromide is compatible with most non-metallic materials of construction such as polypropylene, polyethylene, fiberglass reinforced plastic (FRP), cellulose, cloth, coatings, rubbers, etc. Metals can also be used provided the sodium bromide is kept dry. If the sodium bromide becomes wet, steel will suffer general corrosion and stainless steels and aluminum will suffer pitting attack. The rates of attack will depend upon the amount of oxygen present but in general will not be rapid.
Sodium bromide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2739 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Xinxiang Hongqi District Houyuan Trading Co.,Ltd | +86-0373-3695376 +86-13937349994 | HYJM@houyuanjm.com | China | 300 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | Sara@xmwonderfulbio.com | China | 305 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
View Lastest Price from Sodium bromide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-11 | Sodium bromide
7647-15-6
|
US $0.00 / Kg | 1Kg | 99.0% up | 30 tons per month | Xinxiang Hongqi District Houyuan Trading Co.,Ltd | |
 |
2024-03-11 | Sodium Bromide
7647-15-6
|
US $3500.00-2800.00 / ton | 500ton | 99.9 | 200tons | Shandong Juchuang Chemical Co., LTD | |
 |
2024-03-08 | sodium bromide
7647-15-6
|
US $10.00 / kg | 1kg | 99% | 1000kg | Nantong Guangyuan Chemicl Co,Ltd |
-

- Sodium bromide
7647-15-6
- US $0.00 / Kg
- 99.0% up
- Xinxiang Hongqi District Houyuan Trading Co.,Ltd
-

- Sodium Bromide
7647-15-6
- US $3500.00-2800.00 / ton
- 99.9
- Shandong Juchuang Chemical Co., LTD
-

- sodium bromide
7647-15-6
- US $10.00 / kg
- 99%
- Nantong Guangyuan Chemicl Co,Ltd
7647-15-6(Sodium bromide)Related Search:
1of4