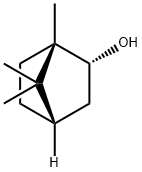
L(-)-Borneol
- CAS No.
- 464-45-9
- Chemical Name:
- L(-)-Borneol
- Synonyms
- L-BORNEOL;[(1S)-ENDO]-(-)-BORNEOL;Linderol;bornel;BORNEOL FLAKES;1-Bornyl alcohol;2-bornanol;BORNEO CAMPHOR;BORNYL ALCOHOL;BORNEOL CRYSTALS
- CBNumber:
- CB3219500
- Molecular Formula:
- C10H18O
- Molecular Weight:
- 154.25
- MDL Number:
- MFCD00003759
- MOL File:
- 464-45-9.mol
- MSDS File:
- SDS
| Melting point | 206-209 °C |
|---|---|
| Boiling point | 210 °C(lit.) |
| alpha | -36.2 º (c=5, C2H5OH) |
| Density | 0.8389 (rough estimate) |
| vapor density | 5.31 (vs air) |
| vapor pressure | 33.5 mm Hg ( 25 °C) |
| FEMA | 2157 | BORNEOL |
| refractive index | -36 ° (C=5, EtOH) |
| Flash point | 150 °F |
| storage temp. | Store below +30°C. |
| solubility | almost transparency in EtOH |
| pka | 15.36±0.60(Predicted) |
| form | Crystalline Powder or Crystals |
| color | White to light yellow |
| Odor | at 10.00 % in dipropylene glycol. pine woody camphor |
| Odor Type | balsamic |
| optical activity | [α]20/D 35.3°, c = 5 in ethanol |
| Water Solubility | INSOLUBLE |
| Merck | 14,1338 |
| BRN | 3587558 |
| LogP | 2.75 at 20℃ |
| CAS DataBase Reference | 464-45-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 1Y84986J9Q |
| NIST Chemistry Reference | Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, (1S-endo)-(464-45-9) |
| EPA Substance Registry System | (-)-Borneol (464-45-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H228 | |||||||||
| Precautionary statements | P210 | |||||||||
| Hazard Codes | F,Xi | |||||||||
| Risk Statements | 11-43 | |||||||||
| Safety Statements | 16-36/37 | |||||||||
| RIDADR | UN 1312 4.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DT5095000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29061900 | |||||||||
| Toxicity | LD50 orally in Rabbit: 5800 mg/kg | |||||||||
| NFPA 704 |
|
L(-)-Borneol price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.14472 | (1S)-(-)-Borneol forsynthesis | 464-45-9 | 25g | $105 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR2429 | D-Camphor Impurity J, endo-borneol Pharmaceutical Secondary Standard; Certified Reference Material | 464-45-9 | 100MG | $534 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHL89583 | (−)-Borneol phyproof? Reference Substance | 464-45-9 | 100MG | $262 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.14472 | (1S)-(-)-Borneol forsynthesis | 464-45-9 | 100g | $151 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.14472 | (1S)-(-)-Borneol forsynthesis | 464-45-9 | 250g | $154 | 2024-03-01 | Buy |
L(-)-Borneol Chemical Properties,Uses,Production
Description
Borneol is an analgetic, antibacterial, and resuscitation-inducing norborneol derived from fresh branches and leaves of Cinnamomum camphora (L.) Presl. Far more than 2000?years ago, it has been introduced to China . In China, it has been firstly recorded in Ming Yi Bie Lu and then included in Tang Ben Cao. It was recorded in history that borneol was derived from Dryobalanops camphora gaertner and then precipitated from the resin to form the natural crystal compound or distilled from the trunk and cooled down to form the crystal compound, which is certified from Indonesia. In China, the natural borneol mainly relied on imports. In recent years, it was extracted from the Lauraceae plants, including Cinnamomum camphora, Cinnamomum longepaniculatum, and Cinnamomum burmannii, which greatlyincreases the resources of natural borneol for China. Cinnamomum camphora is mainly distributed in Jiangxi and Fujian provinces with 81.78% of borneol. Cinnamomum longepaniculatum is mainly distributed in Hunan and Sichuan provinces with 77.57% of borneol. Cinnamomum burmannii is mainly distributed in Yunnan and Guangxi provinces with 70.81% of borneol. Among them, Cinnamomum camphora contains more borneol than the other two types .
Chemical Properties
white to light yellow crystalline powder or
Physical properties
Appearance: colorless to white lumps. Odor: pungent, camphor-like. Density:1.011?g/ cm3 (20?°C). Melting point: 208?°C (406?°F; 481?K). Boiling point: 213?°C (415?°F; 486?K). Solubility: slightly soluble in water (D-form), soluble in chloroform, ethanol, acetone, ether, benzene, toluene, decalin, and tetralin. Flash point: 65? °C (149?°F; 338?K). It’s stable under sealed condition while volatile in the air.
History
Borneol has been widely used worldwide. It has been systematically studied since 1803 in Dutch literature. This might be because borneol was originated from Indonesia which had been the colony of the Netherlands since the seventeenth century. Stockman reviewed the borneol systematically and conducted the preliminary pharmacological experiments. The current pharmacological studies of borneol focus on crossing blood-brain barrier and its mechanism, as well as promoting the penetration of blood-brain barrier after compatibility with other drugs, which has been started by Qizhong Mo in Shanghai Institute of Materia Medica, Chinese Academy of Sciences since 1982 . Qide Wu et al. synthesized a series of ester derivatives of natural borneol and studied its biological properties. It was found that (+) – 4-methoxybenzoic acid borneol ester had a significant effect on the opening of the blood-brain barrier and was less toxic than borneol . Because of the unique chemical structure of borneol and relatively low molecular weight, borneol is often modified to observe whether the drug has such pharmacological effects of antitumor, increasing the penetration of blood-brain barrier, antibacterial, antioxidant, and others. Up to date, there is no druggability report based on borneol modification.
Uses
(-)-Borneol has been used to study its antiapoptotic, antioxidative and neuroprotective effect in human neuroblastoma cells (SH-SY5Y).
Uses
(-)-Borneol is used to prepare its esters by reacting with acids. Its derivatives are used as chiral ligands in asymmetric synthesis. It is also used in flavors and perfumes. Further, it is used in traditional Chinese medicine as moxa. In addition to this, it is used as a component of many essential oils and also used as a natural insect repellent.
Definition
ChEBI: (-)-borneol is a borneol. It is an enantiomer of a (+)-borneol.
Indications
The main efficacy of borneol is to induce resuscitation (with aromatic stimulation), clear stagnated fire (fever feeling), remove nebula for improving eyesight, and relieve swelling and pain. The indications of borneol are sore throat, aphthous, red eyes, purulent ear discharge, convulsions, febrile delirium, sudden faint due to qi depression, stroke, and coma. In Chinese traditional medicine, the borneol is often used as an envoy drug and combined with other drugs but is not used as a single medicine with the inexact efficacy
General Description
(-)-Borneol is an enantiomer. It is a bicyclic monoterpene compound used gengrally for analgesia and anaesthesia. It is considered as positive modulators of GABA receptors.
Flammability and Explosibility
Flammable
Pharmacology
The main pharmacological effects of borneol include anti-inflammatory, antibacterial, central nervous system, and antifertility effects . Guangchi Jiang found that intraperitoneal injection of borneol at 3.5 mL/kg can significantly inhibit foot swelling caused by egg white in rats. Borneol can inhibit and kill Staphylococcus aureus, B-type Streptococcus, and other five common cells with the minimum inhibitory concentration (MIC) of 1.0–2.0% and the lowest bactericidal concentration (MFC) of 1.5–2.0%. There was significant odinopoeia effect on the late pregnant mice after given 112 mg/kg borneol. Qide Liu et al. found that 10% borneol paraffin oil at the dose of 1 mg/kg by oral gavage can significantly increase the concentration of gentamicin in rat brain tissue, suggesting that borneol can change the blood-brain barrier permeability. The current mechanisms of anti-inflammatory effects include inhibition of inflammatory factors of interleukin-1β, tumor necrosis factor-α, and cell adhesion molecule-1 expression. The mechanisms of central nervous system effects are involved in inhibiting p-glycoprotein, opening the intercellular tight junction, increasing the number of pinocytotic vesicles, and improving the phospholipid molecule arrangement of epithelial cell membrane. In addition, borneol also affects the level of nitric oxide and inhibits the elevation of Ca2+ concentration.
Clinical Use
As a traditional Chinese medicine, borneol is commonly used as envoy drugs in the compatibility of traditional Chinese medicine. On behalf of combination drugs such as Danshen dripping pills, Niu Huang Jie Du pills, and watermelon cream, its effect is significant because of its special aromatic smell. Borneol with a certain irritation, oral administration may cause the gastrointestinal discomforts, severely causes vomiting and other adverse reactions.
Safety Profile
Mddly toxic by ingestion. A skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALCOHOLS.
L(-)-Borneol Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | sales@pioneerbiotech.com | China | 3000 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 | ft-sales@nature-standard.com | CHINA | 1923 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | 1015@dideu.com | China | 2263 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8349 | 58 |
View Lastest Price from L(-)-Borneol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-02-24 | L(-)-Borneol
464-45-9
|
US $0.00 / mg | 5mg | ≥97%(HPLC) | 10 g | Shanghai Standard Technology Co., Ltd. | |
 |
2022-05-12 | L(-)-Borneol
464-45-9
|
US $1.10 / g | 1g | 99.0% Min | 100 Tons | Dideu Industries Group Limited | |
 |
2020-05-04 | L(-)-Borneol
464-45-9
|
US $0.00-0.00 / KG | 1KG | 99.0% | 600 Tons | Shaanxi Dideu Medichem Co. Ltd |
-

- L(-)-Borneol
464-45-9
- US $0.00 / mg
- ≥97%(HPLC)
- Shanghai Standard Technology Co., Ltd.
-

- L(-)-Borneol
464-45-9
- US $1.10 / g
- 99.0% Min
- Dideu Industries Group Limited
-

- L(-)-Borneol
464-45-9
- US $0.00-0.00 / KG
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
464-45-9(L(-)-Borneol)Related Search:
1of4