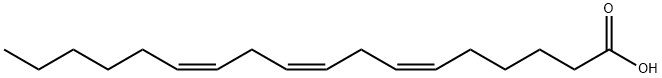
gamma-Linolenic acid
- CAS No.
- 506-26-3
- Chemical Name:
- gamma-Linolenic acid
- Synonyms
- gamolenic acid;C06426;Gamolenic;18:3(n-6);Flunarizin;Flunarizina;R- flax acid;Flunarizinum;γ-linolenicaci;G-LINOLENICACID
- CBNumber:
- CB3238150
- Molecular Formula:
- C18H30O2
- Molecular Weight:
- 278.43
- MDL Number:
- MFCD00065718
- MOL File:
- 506-26-3.mol
- MSDS File:
- SDS
| Melting point | -12~-11℃ |
|---|---|
| Boiling point | 379.5±11.0 °C(Predicted) |
| Density | 0.92 |
| refractive index |
n |
| Flash point | 110 °C |
| storage temp. | -20°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| form | liquid |
| pka | 4.74±0.10(Predicted) |
| color | Colourless |
| Merck | 14,5507 |
| BRN | 1712253 |
| Stability | Light Sensitive |
| InChIKey | VZCCETWTMQHEPK-QNEBEIHSSA-N |
| LogP | 6.570 (est) |
| CAS DataBase Reference | 506-26-3(CAS DataBase Reference) |
| FDA UNII | 78YC2MAX4O |
| NIST Chemistry Reference | Gamolenic acid(506-26-3) |
| ATC code | D11AX02,D11AX52 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 8-10-23 | |||||||||
| HS Code | 29161500 | |||||||||
| NFPA 704 |
|
gamma-Linolenic acid price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 62174 | γ-Linolenic acid analyticalstandard | 506-26-3 | 100mg | $226 | 2024-03-01 | Buy |
| Sigma-Aldrich | 62174 | γ-Linolenic acid analyticalstandard | 506-26-3 | 500mg | $624 | 2024-03-01 | Buy |
| TCI Chemical | L0152 | γ-Linolenic Acid >98.0%(GC)(T) | 506-26-3 | 100mg | $98 | 2024-03-01 | Buy |
| TCI Chemical | L0152 | γ-Linolenic Acid >98.0%(GC)(T) | 506-26-3 | 1g | $485 | 2024-03-01 | Buy |
| Cayman Chemical | 90220 | γ-Linolenic Acid ≥98% | 506-26-3 | 50mg | $25 | 2024-03-01 | Buy |
gamma-Linolenic acid Chemical Properties,Uses,Production
Description
γ-Linolenic acid (gamma-linolenic acid or GLA, INN and USAN gamolenic acid) is a fatty acid found primarily in vegetable oils. It is sold as a dietary supplement for treating problems with inflammation and auto-immune diseases, although its efficacy is disputed.
Description
γ-
Chemical Properties
GLA is categorized as an n?6 (also called ω?6 or omega-6) fatty acid, meaning that the first double bond on the methyl end (designated with n or ω) is the sixth bond. In physiological literature, GLA is designated as 18:3 (n?6). GLA is a carboxylic acid with an 18-carbon chain and three cis double bonds. It is an isomer of α-linolenic acid, which is a polyunsaturated n?3 (omega-3) fatty acid, found in rapeseed canola oil, soy beans, walnuts, flax seed (linseed oil), perilla, chia, and hemp seed.
Occurrence
GLA is obtained from vegetable oils such as GLA safflower oil (Carthamus tinctorius), evening primrose (Oenothera biennis) oil, blackcurrant seed oil, borage oil, and hemp seed oil. GLA is also found in edible hemp seeds, oats, barley, and spirulina. Each contains varying amounts of the fatty acid, with GLA safflower oil at 40% GLA being a novel concentrated form. This is a new genetically modified oil and has been available in commercial quantities since 2011. It should be noted that conventional safflower oils have zero GLA. Borage oil ranges from 15 % to 20 % and evening primrose oil ranges from 8 % to 10 % GLA.
The human body produces GLA from linoleic acid (LA). This reaction is catalyzed by Δ6 - desaturase (D6D), an enzyme that allows the creation of a double bond on the sixth carbon counting from the carboxyl terminus. LA is consumed sufficiently in most diets, from such abundant sources as cooking oils and meats. However, a lack of GLA can occur when there is a reduction of the efficiency of the D6D conversion (for instance, as people grow older or when there are specific dietary deficiencies) or in disease states wherein there is excessive consumption of GLA metabolites.
History
GLA was first isolated from the seed oil of evening primrose. This herbal plant was grown by Native Americans to treat swelling in the body. In the 17th century, it was introduced to Europe and became a popular folk remedy, earning the name king's cure-all. in 1919, Heiduschka and Lüft extracted the oil from evening primrose seeds and described an unusual linolenic acid, which they name γ-. Later, the exact chemical structure was characterized by Riley.
Although there are α- and γ- forms of linolenic acid, there is no β- form. One was once identified, but it turned out to be an artifact of the original analytical process.
Uses
γ-linolenic acid has been used as an analytical standard in gas chromatography. It may be used in nutritional studies regarding weight regain and as a possible tumor suppression agent. γ-linolenic acid is used in studies on the mechanisms and prevention of oxidation/peroxidation of unsaturated fatty acids.
Uses
γ-Linolenic Acid is a fatty acid found mainly in vegetable oil. Studies suggest that γ-Linolenic Acid may have immune regulating and anti-inflammatory effects. γ-Linolenic Acid has also been used in the treatment of atopic eczeme although its efficacy is
Definition
ChEBI: A C18, omega-6 acid fatty acid comprising a linolenic acid having cis- double bonds at positions 6, 9 and 12.
Biochem/physiol Actions
Gamma-linolenate (C18:6,9,12) differs from α-linolenate (C18:9,12,15) in the positions of the double bonds.
References
[1] yagaloff k a, franco l, simko b, et al. essential fatty acids are antagonists of the leukotriene b4 receptor[j]. prostaglandins, leukotrienes and essential fatty acids, 1995, 52(5): 293-297.
[2] crooks s w, stockley r a. leukotriene b4[j]. the international journal of biochemistry & cell biology, 1998, 30(2): 173-178.
[3] tasset-cuevas i, fernández-bedmar z, lozano-baena m d, et al. protective effect of borage seed oil and gamma linolenic acid on dna: in vivo and in vitro studies[j]. plos one, 2013, 8(2): e56986.
[4] jamal g a, carmichael h. the effect of γ‐linolenic acid on human diabetic peripheral neuropathy: a double‐blind placebo‐controlled trial[j]. diabetic medicine, 1990, 7(4): 319-323.
[5] andreassi m, forleo p, dilohjo a, et al. efficacy of γ-linolenic acid in the treatment of patients with atopic dermatitis[j]. journal of international medical research, 1997, 25(5): 266-274.
gamma-Linolenic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 290 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 2995 | 58 |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | sales@pioneerbiotech.com | China | 3000 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
Related articles
- Uses in Cosmetics of gamma-Linolenic acid
- GLA-rich vegetable oils have been evaluated in a variety of clinical conditions. The oils have been reported to have a skin ba....
- Mar 3,2022
- Metabolism and Cutaneous Significance of Linoleic and Gamma-Linolenic Acid
- Epidermal hyperproliferation and increased water loss through the skin are lead symptoms in experimental and clinical fatty ac....
- Mar 3,2022
View Lastest Price from gamma-Linolenic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Gamma Linolenic Acid Powder(GLA)
506-26-3
|
US $0.00 / kg | 1kg | 10% 12% 20% | 3000 kg | BINBO BIOLOGICAL CO.,LTD | |
 |
2023-07-27 | gamma-Linolenic acid
506-26-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-02-23 | gamma-Linolenic acid
506-26-3
|
US $34.00 / kg | 1kg | 99% | 5000 tons | Hebei Duling International Trade Co. LTD |
-

- Gamma Linolenic Acid Powder(GLA)
506-26-3
- US $0.00 / kg
- 10% 12% 20%
- BINBO BIOLOGICAL CO.,LTD
-

- gamma-Linolenic acid
506-26-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- gamma-Linolenic acid
506-26-3
- US $34.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD