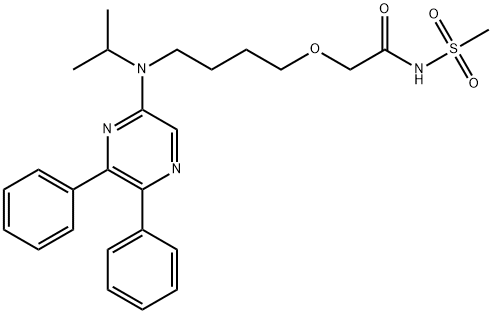
Selexipag
- CAS No.
- 475086-01-2
- Chemical Name:
- Selexipag
- Synonyms
- Selexipag;Slipper;NS-304;elexipag;Selxxipag;ACT-293987;Selexipag API;The company west;NS-304(Selexipag);Selexipag(NS-304)
- CBNumber:
- CB32454222
- Molecular Formula:
- C26H32N4O4S
- Molecular Weight:
- 496.62
- MDL Number:
- MFCD10567093
- MOL File:
- 475086-01-2.mol
| Melting point | 134-138°C |
|---|---|
| Density | 1.210±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 3.82±0.40(Predicted) |
| form | Solid |
| color | Pale Yellow |
| FDA UNII | 5EXC0E384L |
| ATC code | B01AC27 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 | |||||||||
| NFPA 704 |
|
Selexipag price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 10010411 | NS-304 ≥98% | 475086-01-2 | 1mg | $45 | 2024-03-01 | Buy |
| Cayman Chemical | 10010411 | NS-304 ≥98% | 475086-01-2 | 5mg | $198 | 2024-03-01 | Buy |
| Cayman Chemical | 10010411 | NS-304 ≥98% | 475086-01-2 | 10mg | $351 | 2024-03-01 | Buy |
| Cayman Chemical | 10010411 | NS-304 ≥98% | 475086-01-2 | 50mg | $1528 | 2024-03-01 | Buy |
| Tocris | 3351 | Selexipag ≥98%(HPLC) | 475086-01-2 | 50 | $1072 | 2021-12-16 | Buy |
Selexipag Chemical Properties,Uses,Production
Description
Selexipag and its active metabolite, the corresponding carboxylic acid, are nonprostanoid prostaglandin I2 (PGI-2) receptor agonists. The N-methylsulfonamide within selexipag is hydrolyzed to the corresponding carboxylic acid in vivo by hepatic microsomes at a rate which provides a slow-release pharmacological effect. The compound was originally discovered by Nippon Shinyaki and later licensed to Actelion for development. The drug was approved in 2015 and first launched for the oral treatment of pulmonary arterial hypertension (PAH) in the U.S. in 2016 to delay disease progression and reduce the risk of hospitalization.
Uses
Selexipag is an orally available, highly selective, long-acting prostacyclin (IP) receptor agonist prodrug. It is a potential drug for the treatment of various vascular disorders such as pulmonary arterial hypertension and arteriosclerosis obliterans.
Definition
ChEBI: Selexipag is a member of the class of pyrazines that is N-(methanesulfonyl)-2-{4-[(propan-2-yl)(pyrazin-2-yl)amino]butoxy}acetamide carrying two additional phenyl substituents at positions 5 and 6 on the pyrazine ring. An orphan drug used for the treatment of pulmonary arterial hypertension. It is a prodrug for ACT-333679 (the free carboxylic acid). It has a role as an orphan drug, a prostacyclin receptor agonist, a platelet aggregation inhibitor, a vasodilator agent and a prodrug. It is a monocarboxylic acid amide, an ether, a member of pyrazines, an aromatic amine, a tertiary amino compound and a N-sulfonylcarboxamide. It is functionally related to an ACT-333679.
Biological Activity
Prostaglandin I2 (PGI2) is a potent vasorelaxant and inhibitor of human platelet aggregation that mediates its actions by binding to a specific G protein- coupled receptor, the IP receptor, on the surface of endothelial cells and platelets. The IP receptor also participates in signal transduction of the pain response, cardioprotection, and inflammation. Selexipag(NS-304) is a prodrug of the active form of MRE-269, which is a potent and selective agonist for the human IP receptor with a Ki value of 20 nM. In contrast to prostaglandin I2, which has a half-life of 30 seconds to a few minutes in vivo, NS-304 is long-acting. Plasma concentrations of MRE-269 remain near peak levels for more than eight hours in rats and dogs after NS-304 was administered orally.
Clinical Use
Selexipag was approved by the United States FDA on December 22, 2015 for the treatment of pulmonary arterial hypertension (PAH) to delay disease progression and reduce risk of hospitalization.
Selective IP receptor agonist:
Treatment of pulmonary arterial hypertension.
Synthesis
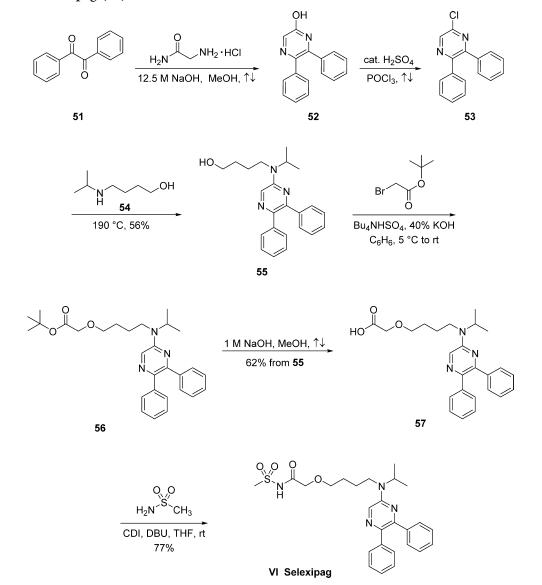
The synthesis of selexipag began with condensation of commercially available benzil (51) and glycinamide hydrochloride in the presence of concentrated sodium hydroxide in refluxing MeOH to yield hydroxypyrazine 52. This compound was subsequently converted to 5-chloro-2,3-diphenylpyrazine (53) upon treatment with refluxing POCl3 in the presence of a catalytic amount of H2SO4. Chloride 53 was then subjected to neat 4-(isopropylamino)-1-butanol (54, prepared by the reductive alkylation of 4-amino-1-butanol and acetone with hydrogen over PtO2 in EtOH) at 190 ??C to give aminopyrazinyl alcohol 55 in 56% yield as colorless crystals. Alcohol 55 was alkylated with tert-butyl bromoacetate using Bu4NHSO4 as a phase-transfer catalyst and 40% aqueous KOH in benzene to give ester 56. Although it is particularly unusual to employ benzene on a production scale, these are the only reported conditions for this transformation. The crude ester 56 was then saponified using methanolic sodium hydroxide to yield the corresponding carboxylic acid 57 in 62% as pale-yellow crystals in two steps from compound 55. Finally, the carboxylic acid 57 was coupled with methanesulfonamide in the presence of CDI and DBU in THF to give selexipag (VI) in 77% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly reduced by
rifampicin - consider increasing selexipag dose
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin -
consider increasing selexipag dose.
Clopidogrel: concentration of selexipag possibly
increased - consider reducing dose of selexipag.
Deferasirox: concentration of selexipag possibly
increased - consider reducing dose of selexipag.
Lipid-lowering drugs: concentration possibly
increased by gemfibrozil - avoid.
Teriflunomide: concentration of selexipag possibly
increased - consider reducing dose of selexipag
Metabolism
Selexipag is rapidly absorbed and is hydrolysed by
CES1 in the liver to its active metabolite. Oxidative
metabolism catalysed by CYP3A4 and CYP2C8 leads
to the formation of hydroxylated and dealkylated
products. UGT1A3 and UGT2B7 are involved in the
glucuronidation of the active metabolite.
Excretion is mainly via the faeces (93%) and 12% via the
urine.
storage
Store at -20°C
Selexipag Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Seasons Biotechnology Co., Ltd. | +86-0576-89232655 +86-13566878689 | info@seasonsbio.com | China | 46 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9352 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Beijing Yibai Biotechnology Co., Ltd | 0086-182-6772-3597 | sales04@yibaibiotech.com | CHINA | 419 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Selexipag manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-03-06 | Selexipag
475086-01-2
|
US $10.00 / Kg/Drum | 1KG | 98% | 10 ton | Hebei Guanlang Biotechnology Co,.LTD | |
 |
2021-09-29 | selexipag
475086-01-2
|
US $0.00 / KG | 10g | 99%min | 10kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2021-07-13 | Selexipag
475086-01-2
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |