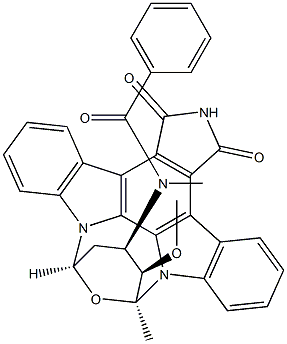
StaupriMide
- CAS No.
- 154589-96-5
- Chemical Name:
- StaupriMide
- Synonyms
- Stauprimide;Stauprimide-d5;benzaMide deriv.;N-Benzoyl-7-oxostaurosporine;Stauprimide - CAS 154589-96-5 - Calbiochem;N-[(9S,10R,11R,13R)-2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-1,3-dioxo-9,13-epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-11-yl]-N-methyl benzamide;Benzamide, N-[(9S,10R,11R,13R)-2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-1,3-dioxo-9,13-epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-11-yl]-N-methyl-;[9S-(9α,10β,11β,13α)]-N-(2,3,10,11,12,13-Hexahydro-10-methoxy-9-methyl-1,3-dioxo-9,13-epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-11-yl)-N-methylbenzamide;N-((5S,6R,7R,9S)-6-Methoxy-5-methyl-14,16-dioxo-6,7,8,9,15,16-hexahydro-5H,14H-17-oxa-4b,9a,15-triaza-5,9-methanodibenzo[b,h]cyclonona[jkl]cyclopenta[e]-as-indacen-7-yl)-N-methylbenzamide
- CBNumber:
- CB32518818
- Molecular Formula:
- C35H28N4O5
- Molecular Weight:
- 584.62
- MDL Number:
- MFCD14635443
- MOL File:
- 154589-96-5.mol
- MSDS File:
- SDS
| Density | 1.52±0.1 g/cm3(Predicted) |
|---|---|
| storage temp. | -20°C |
| solubility | DMSO: >20mg/mL |
| pka | 9.51±0.70(Predicted) |
| form | Yellow solid |
| color | white to beige |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
| FDA UNII | T7R6VV8WUL |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P271-P280 |
| WGK Germany | 3 |
| HS Code | 2934999090 |
StaupriMide price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S2951 | Stauprimide ≥98% (HPLC) | 154589-96-5 | 1mg | $214 | 2024-03-01 | Buy |
| Sigma-Aldrich | 569393 | Stauprimide - CAS 154589-96-5 - Calbiochem | 154589-96-5 | 500μg | $246 | 2024-03-01 | Buy |
| Cayman Chemical | 15398 | Stauprimide ≥98% | 154589-96-5 | 500μg | $50 | 2024-03-01 | Buy |
| Cayman Chemical | 15398 | Stauprimide ≥98% | 154589-96-5 | 1mg | $85 | 2024-03-01 | Buy |
| Sigma-Aldrich | S2951 | Stauprimide ≥98% (HPLC) | 154589-96-5 | 5mg | $855 | 2024-03-01 | Buy |
StaupriMide Chemical Properties,Uses,Production
Description
Stauprimide is a small molecule that increases the efficiency of mouse and human embryonic stem cell differentiation in conjunction with lineage specification cues (EC50 = 0.3 μM). It specifically inhibits the nuclear localization of NME2, which results in the suppression of c-
Uses
Stauprimide is a semi-synthetic analogue of the staurosporine family of indolocarbazoles. Stauprimide was first published in 1994 as part of an extensive structure-activity investigation to improve the selective inhibition of protein kinase C as potential antitumour agents. Stauprimide increases the efficiency of the directed differentiation of mouse and human embryonic stem cells in synergy with defined extracellular signalling cues. Stauprimide interacts with NME2 (PUF) transcription factor to down-regulate c-Myc expression, leading to differentiation of stem cells.
Uses
Stauprimide is an enhancer stem cell differentiation into endoderm. In vitro differentiation of embryonic stem cells is of great interest to regenerative medicine, and current protocols are labor-intensive and expensive. Small molecules that induce or enhance differentiation are highly desired. High-content screening identified compounds that enhance endodermal differentiation in the presence of low levels of Activin A. Stauprimide increased definitive endodermal markers but not markers for visceral/parietal endoderm or mesoderm. Stauprimide-differentiated cells could be further differentiated into hepatocytes. Stauprimide treatment during differentiation decreased the concentration of Activin A required for definitive endoderm formation, and it eliminated the need for serum. The mechanism of action of stauprimide is to sensitize cells for differentiation. Stauprimide enabled differentiation into other cell lineages under varying differentiation conditions, including neurons, hematopoietic mesoderm, beating cardiac myocytes, and skeletal muscle.
Uses
Labelled Stauprimide (S684700). It is an enhancer stem cell differentiation into endoderm.
General Description
A cell-permeable ESCs (Embryonic Stem Cells) EMT (Epithelial-Mesenchymal Transition) inducer that is reported to prime/sensitize human and murine ESC cultures for much more enhanced/efficient differentiation into progenitor cells of multiple lineages, including Activin A- (Cat. No. 114700) induced definitive endoderm differentiation (60% differentiation from murine R1 ESCs with 5 ng/ml Activin A and 200 nM Spd), ATRA- (Cat. No. 554720) induced neuronal progenitor differentiation, and BMP-4- (Cat. No. 203642) induced mesoderm differentiation, under suboptimal lineage specifying stimuli concentrations and often serum-free conditions, while Spd sensitization alone does not induce any ESCs differentiation. Spd is shown to interact with NME2/DNPK B/PUF and prevent NME2 nuclear localization and c-myc transcription in murine R1 ESC cultures (46% and 85% drop in c-myc mRNA in 24 h, respectively, with 200 nM and 500 nM Spd), which accounts for, at least in part, its EMT induction activity. Although a structural analog of the broad-spectrum kinase inhibitors Staurosporine (Cat. Nos. 569396 and 569397) and UCN-01 (Cat. No. 539644), Spd exhibits significant kinase inhibitory activities only at elevated concentrations, but not at concentrations ≤500 nM typically used in EMT induction.
Biochem/physiol Actions
Stauprimide is an enhancer stem cell differentiation into endoderm. In vitro differentiation of embryonic stem cells is of great interest to regenerative medicine, and current protocols are labor-intensive and expensive. Small molecules that induce or enhance differentiation are highly desired. High-content screening identified compounds that enhance endodermal differentiation in the presence of low levels of Activin A. Stauprimide increased definitive endodermal markers but not markers for visceral/parietal endoderm or mesoderm. Stauprimide-differentiated cells could be further differentiated into hepatocytes. Stauprimide treatment during differentiation decreased the concentration of Activin A required for definitive endoderm formation, and it eliminated the need for serum. The mechanism of action of stauprimide is to sensitize cells for differentiation. Stauprimide enabled differentiation into other cell lineages under varying differentiation conditions, including neurons, hematopoietic mesoderm, beating cardiac myocytes, and skeletal muscle. The cellular target of stauprimide was determined to be inhibition of NME2 transcription factor translocation to the nucleus, leading to down-regulation of c-Myc expression.
storage
-20°C
References
1) Zhu et al. (2009), A small molecule primes embryonic stem cells for differentiation; Cell Stem Cell, 4 416 2) Zaret et al. (2009), Using small molecules to great effect in stem cell differentiation; Cell Stem Cell, 4 373 3) Bouvard et al. (2017) Small molecule selectively suppresses MYC transcription in cancer cells; Proc. Natl. Acad. Sci. USA 114 3497
StaupriMide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 20000 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Aurisco Pharmaceutical (Tianjin) Inc. | 022-59885855 | liuyujie@aurisco.com | China | 96 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| Chemsky (shanghai) International Co.,Ltd | 021-50135380 | shchemsky@sina.com | China | 15421 | 60 |
| Shandong Xiya Chemical Co., Ltd | 13355009207 13355009207 | 3007715519@qq.com | China | 18739 | 57 |