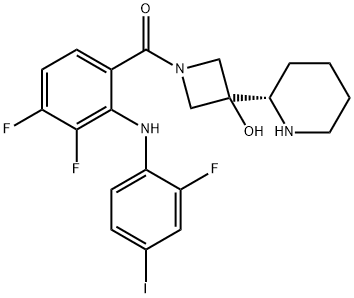
XL518
- CAS No.
- 934660-93-2
- Chemical Name:
- XL518
- Synonyms
- CobiMetinib;GDC-0973;XL518;RG7420;CS-1977;Cobimetinib-D4;GDC-0973/RG7420;XL518 ,GDC-0973;XL518 USP/EP/BP;GDC-0973(XL-518)
- CBNumber:
- CB32551361
- Molecular Formula:
- C21H21F3IN3O2
- Molecular Weight:
- 531.31
- MDL Number:
- MFCD22124461
- MOL File:
- 934660-93-2.mol
| Melting point | 165 - 166°C |
|---|---|
| Boiling point | 565.9±50.0 °C(Predicted) |
| Density | 1.706 |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| pka | 13.13±0.20(Predicted) |
| form | Solid |
| color | Off-White |
| FDA UNII | ER29L26N1X |
| ATC code | L01EE02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| HS Code | 29333990 | |||||||||
| NFPA 704 |
|
XL518 price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 19563 | Cobimetinib ≥98% | 934660-93-2 | 1mg | $49 | 2024-03-01 | Buy |
| Cayman Chemical | 19563 | Cobimetinib ≥98% | 934660-93-2 | 5mg | $179 | 2024-03-01 | Buy |
| Cayman Chemical | 19563 | Cobimetinib ≥98% | 934660-93-2 | 10mg | $333 | 2024-03-01 | Buy |
| Cayman Chemical | 19563 | Cobimetinib ≥98% | 934660-93-2 | 25mg | $711 | 2024-03-01 | Buy |
| TRC | X746250 | XL518 | 934660-93-2 | 10mg | $275 | 2021-12-16 | Buy |
XL518 Chemical Properties,Uses,Production
Description
Cobimetinib, codeveloped by Genentech and Exelixis, was approved in August 2015 in Switzerland and November 2015 in the U.S. and Europe for the treatment of unresectable or metastatic BRAFV600 mutationpositive melanoma when used in combination with vemurafenib. Cobimetinib is a potent, highly selective reversible inhibitor of mitogen-activated protein kinases (MEK) 1 and 2,120 which serves to inhibit phosphorylation of ERK1/2,121 disrupting the MAPK pathway which is responsible for cell proliferation, cell survival, and migration.122 Combination of cobimetinib with vemurafenib, an important BRAF inhibitor,123 enables targeting of multiple points on the MAPK pathway, leading to overall enhanced tumor cell apoptosis and response as compared to stand-alone treatment with vemurafenib.124 Specifically, in a representative trial of previously untreated patients with BRAFV600 mutation-positive, unresectable, stage IIIc or IV melanoma, combination of these two therapies led to a significantly improved progression-free survival and overall response rate versus patients treated only with vemurafenib.
Uses
A potent, selective, orally bioavailable inhibitor of MEK1, a component of the RAS/RAF/MEK/ERK pathway. It inhibits proliferation and stimulates apoptosis in a variety of human tumor cell lines. In preclinical xenograft models, oral administration of XL518 results in sustained inhibition of pERK in tumor tissue, but not brain tissue, leading to tumor growth inhibition and regression at well tolerated doses.
Definition
ChEBI: Cobimetinib is a member of the class of N-acylazetidines obtained by selective formal condensation of the carboxy group of 3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzoic acid with the secondary amino group from the azetidine ring of 3-[(2S)-piperidin-2-yl]azetidin-3-ol. An inhibitor of mitogen-activated protein kinase that is used (as its fumarate salt) in combination with vemurafenib for the treatment of patients with unresectable or metastatic melanoma. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor and an antineoplastic agent. It is a member of piperidines, a N-acylazetidine, a tertiary alcohol, an aromatic amine, a secondary amino compound, a difluorobenzene and an organoiodine compound. It is a conjugate base of a cobimetinib(1+).
Clinical Use
Protein kinase inhibitor:
Treatment of unresectable or metastatic melanoma
with a BRAF V600 mutation in combination with
vemurafenib
Synthesis
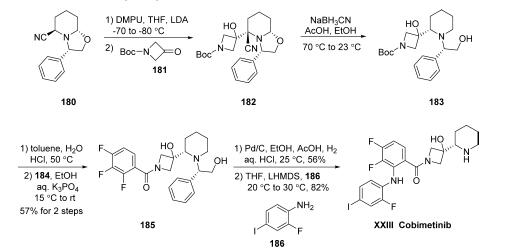
Structurally, cobimetinib features an interesting azetidinol substructure appended to the 2-position of a piperidine, rendering the 2-carbon of the piperidine as a stereogenic center bearing the (S)-configuration. While the early discovery routes to cobimetinib relied on a piperidine resolution-based route for accessing the cobimetinib core, the scale route to this drug employs an impressive N-cyanomethyl oxazolidine chiral auxiliary-mediated sequence to induce strereocontrol, generating the requisite stereocenter with excellent selectivity and requiring no chromatographic purification in the overall synthetic sequence. Toward this end, the most likely scale synthetic approach was initiated with deprotonation of commercially available (3S,5R,8aS)-3-phenyl-hexahydrooxazolo[ 3,2-a]pyridine-carbonitrile (180), followed by addition of commercial 3-oxo-azetidine-1-carboxylic acid tert-butyl ester (181), yielded 182 in high purity (92%) after distillation and providing a rapid route to the core structure of cobimetinib. One-pot ring opening and reduction of 182 was accomplished by exposing this hemiaminal to acetic acid and sodium cyanoborohydride, giving rise to intermediate 183. This carbamate could be further reacted with aqueous HCl in toluene to liberate the azetidine amine salt in high purity (97.6%), which underwent immediate acylation with commercially available 2,3,4-trifluoro-benzoyl chloride (184) to enable formation of intermediate 185 in 85% purity after aqueous workup. Reductive cleavage of the chiral auxiliary of 185 with Pd/C and H2 under aqueous acidic conditions (AcOH, aq HCl) yielded the desired piperidine amine, which could be isolated as a solid (99.6% pure) after trituration with aqueous HCl. Finally, aromatic fluoride substitution with commercially available aniline 186 under basic conditions provided cobimetinib in 99.7% purity after slow precipitation from toluene (it is important to note that the authors offer no comment as to the regioselectivity of this aromatic substitution reaction). While the drug reportedly exists as a fumarate salt, no synthetic reports describing the conversion of cobimetinib to the corresponding fumarate salt were available in the chemical literature to our knowledge at the time of publication.
Drug interactions
Potentially hazardous interactions with other drugs
Antifungals: concentration increased by itraconazole.
Antipsychotics: increased risk of agranulocytosis -
avoid.
Metabolism
Metabolised by oxidation by CYP3A and glucuronidation by UGT2B7. Extensively metabolised and eliminated in faeces
XL518 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shanghai VastPro Technology Development Co., Ltd. | 021-20608178 18930468532 | sales@vastprotech.com | CHINA | 12 | 58 |
| Shanghai Rochi Pharmaceutical Co.,Ltd. | 21-38751876 +8615000076078 | info@rochipharma.com | China | 431 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29322 | 58 |
View Lastest Price from XL518 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-13 | Cobimetinib
934660-93-2
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-10 | Cobimetinib
934660-93-2
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2020-08-07 | Cobimetinib
934660-93-2
|
US $0.00 / KG | 1g | 99 | 100kg | Shanghai VastPro Technology Development Co., Ltd. |
-

- Cobimetinib
934660-93-2
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Cobimetinib
934660-93-2
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Cobimetinib
934660-93-2
- US $0.00 / KG
- 99
- Shanghai VastPro Technology Development Co., Ltd.