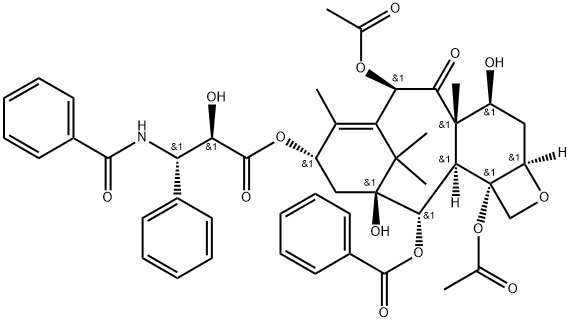
Paclitaxel
- CAS No.
- 33069-62-4
- Chemical Name:
- Paclitaxel
- Synonyms
- TAXOL;Abraxane;BACCATIN III;Paclitaxe;5β,20-Epoxy-1,7β-dihydroxy-9-oxotax-11-ene-2α,4,10β,13α-tetrayl 4,10-diacetate 2-benzoate 13-[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate];[2aR-[2aα,4β,4aβ,6α,9α(αR*,βS*),11α,12α,12aα,12bα]]-β-(Benzoylamino)-α-hydroxy-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-ylesterbenzenepropanoicacid;6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-ylester, (αR, βS)-;αR-hydroxy-βS-(benzoylamino)-benzenepropanoic acid, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester;Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)-;TAXUS
- CBNumber:
- CB3273425
- Molecular Formula:
- C47H51NO14
- Molecular Weight:
- 853.92
- MDL Number:
- MFCD00869953
- MOL File:
- 33069-62-4.mol
- MSDS File:
- SDS
| Melting point | 213 °C (dec.)(lit.) |
|---|---|
| Boiling point | 774.66°C (rough estimate) |
| alpha | D20 -49° (methanol) |
| Density | 0.200 |
| refractive index | -49 ° (C=1, MeOH) |
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| solubility | methanol: 50 mg/mL, clear, colorless |
| form | powder |
| pka | 11.90±0.20(Predicted) |
| color | white |
| Water Solubility | 0.3mg/L(37 ºC) |
| λmax | 227nm(MeOH)(lit.) |
| Merck | 14,6982 |
| BRN | 1420457 |
| Stability | Stable. Incompatible with strong oxidizing agents. Combustible. |
| InChIKey | RCINICONZNJXQF-MZXODVADSA-N |
| LogP | 3.950 (est) |
| FDA 21 CFR | 516.1684 |
| CAS DataBase Reference | 33069-62-4(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | ABI-007; Abraxane; paclitaxel |
| FDA UNII | P88XT4IS4D |
| NCI Drug Dictionary | Abraxane |
| ATC code | L01CD01 |
| Proposition 65 List | Paclitaxel |
| EPA Substance Registry System | Paclitaxel (33069-62-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H317-H318-H334-H335-H340-H360D-H372 | |||||||||
| Precautionary statements | P202-P260-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 37/38-41-42/43-62-68-40-48-20/21/22-68/20/21/22 | |||||||||
| Safety Statements | 22-26-36/37/39-45 | |||||||||
| RIDADR | 1544 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DA8340700 | |||||||||
| F | 10-21 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29329990 | |||||||||
| Toxicity | LD50 intraperitoneal in mouse: 128mg/kg | |||||||||
| NFPA 704 |
|
Paclitaxel price More Price(87)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 580555 | Paclitaxel | 33069-62-4 | 5mg | $125 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHL89806 | Taxol phyproof? Reference Substance | 33069-62-4 | 10MG | $300 | 2024-03-01 | Buy |
| Sigma-Aldrich | 5.08227 | InSolution Paclitaxel, Taxus sp. - CAS 33069-62-4 - Calbiochem | 33069-62-4 | 10mg | $225 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1491332 | Paclitaxel United States Pharmacopeia (USP) Reference Standard | 33069-62-4 | 200mg | $4200 | 2024-03-01 | Buy |
| TCI Chemical | P1632 | Paclitaxel >98.0%(HPLC) | 33069-62-4 | 100mg | $325 | 2024-03-01 | Buy |
Paclitaxel Chemical Properties,Uses,Production
Description
Paclitaxel, a natural product isolated from the bark of the Pacific yew, is effective in treating refractory metastatic ovarian cancer. Unlike any other antineoplastic agents, paclitaxel appears to have several possible mechanisms of action, including an antimicrotubule action through the promotion of tubulin polymerization and stabilization of microtubules, thereby, halting mitosis and promoting cell death. The supply of paclitaxel is limited by its low natural abundance and currently it is being manufactured by a semi-synthetic route from deacetylbaccatin Ⅲ that is isolated from the needles of the yew tree. Recent completion of two total syntheses of taxol conquered the structural complexity of the title compound and may be useful in obtaining certain closely related analogs, some of which have been found to have antitumor activity. Paclitaxel has potential uses in the treatment of metastatic breast cancer, lung cancer, head and neck cancer, and malignant melanoma.
Chemical Properties
White Powder
Physical properties
Appearance: Odorless and tasteless white or kind of white crystal powder. Solubility: Poorly soluble in water but slightly soluble in ether. Soluble in methanol, acetonitrile, chloroform, acetone, and other organic solvents. Melting point: 213–216?°C. Specific optical rotation: ?49° (C?=?1, MeOH); Curl: 20° to D?=?49.0–55.0° (10?mg/mL of methanol solution) in anhydrous dry goods without solvents.
Originator
NIH (U.S.A.)
History
The toxic ingredients in branches and leaves of Taxus chinensis were separated in 1856 and named “taxine,” which was identified as a kind of white alkaloid’s component. Currently, among all the antitumor drugs, the sale of paclitaxel becomes the first in the world as a well-recognized anticancer drug with potent broad-spectrum activity. In October of 1995, China became the second country with formal production of paclitaxel and its injection in the world. The achievement was gained under the unremitting efforts of researchers in the Institute of Materia Medica, Chinese Academy of Medical Sciences.
Uses
Paclitaxel is an antineoplastic that used to treat patients with lung, ovarian, breast cancer, head and neck cancer, and advanc ed forms of Kaposi's sarcoma. Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It is also used in the study of structure and function of microtubles into tubulin.
Preparation
The total synthesis of paclitaxel (Taxol) is described. Double Rubottom oxidation of the bis(silyl enol ether) derived from a tricarbocyclic diketone effectively installed a bridgehead olefin and C-5/C-13 hydroxy groups in a one-step operation. The novel Ag-promoted oxetane formation smoothly constructed the tetracyclic framework of paclitaxel.
Total Synthesis of Paclitaxel
The biosynthesis of paclitaxel involves the condensation of the three isoprenyl diphosphate (IPP) units with dimethylallyl diphosphate (DMAPP). Plants are unique in producing IPP and DMAPP by both the mevalonic pathway (MVA) in the cytosol or via the methylerythritol phosphate (MEP) pathway in the plastids.
Paclitaxel: biosynthesis, production and future prospects
Indications
Paclitaxel (Taxol) is a highly complex, organic compound isolated from the bark of the Pacific yew tree. It binds to tubulin dimers and microtubulin filaments, promoting the assembly of filaments and preventing their depolymerization. This increase in the stability of microfilaments results in disruption of mitosis and cytotoxicity and disrupts other normal microtubular functions, such as axonal transport in nerve fibers. The major mechanism of resistance that has been identified for paclitaxel is transport out of tumor cells, which leads to decreased intracellular drug accumulation. This form of resistance is mediated by the multidrug transporter P-glycoprotein.
Definition
ChEBI: Paclitaxel is a tetracyclic diterpenoid isolated originally from the bark of the Pacific yew tree, Taxus brevifolia. It is a mitotic inhibitor used in cancer chemotherapy. Note that the use of the former generic name 'taxol' is now limited, as Taxol is a registered trade mark. It has a role as a microtubule-stabilising agent, a metabolite, a human metabolite and an antineoplastic agent. It is a tetracyclic diterpenoid and a taxane diterpenoid. It is functionally related to a baccatin III.
brand name
Abraxane (Abraxis); Taxol (Bristol-Myers Squibb).
Therapeutic Function
Antineoplastic
General Description
Paclitaxel (commercial name, Taxol) a complex diterpene alkaloid isnaturally obtained from Taxus species (family Taxaceae). Paclitaxel has been provedas highly effective in the treatment of various types of cancers, since it acts as amicrotubule-stabilizing agent to protect against disassembly. Paclitaxel was developed by the National Cancer Institute, USA, as a drug for cancer therapy andused for the treatment of refractory ovarian cancer, metastatic breast and lung cancer,and Kaposi’s sarcoma (Srivastava et al. 2005). The natural source of paclitaxelis the bark of several Taxus species; however, the cost of extraction is very highsince the concentration of paclitaxel accumulation is very low (0.02% of dry weight)and also entails the destruction of natural resources (Cusido et al. 2014). Eventhough, paclitaxel can be chemically synthesized, but this process is not commerciallyviable. Plant cell cultures have been developed for the production of paclitaxelby Phyton Biotech in 1995, and in 2004 the FDA has approved the use of plantculture supply of paclitaxel/Taxol (Leone and Roberts 2013).
Air & Water Reactions
May be sensitive to prolonged exposure to moisture. .
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Flash point data for Paclitaxel are not available. Paclitaxel is probably combustible.
Biological Activity
Antitumor agent; promotes and stabilizes tubulin polymerization, causing cell cycle arrest. Induces autocatalytic activation of caspase-10 in CCRF-HSB-2 cells, triggering apoptosis.
Biochem/physiol Actions
Product does not compete with ATP.
Mechanism of action
Paclitaxel is currently the only known drug that can promote microtubule polymerization and stabilize polymerized microtubules. It can only form on polymerized microtubules and does not react with non-polymerized microtubule protein dipolymers. After coming in contact with paclitaxel, cells will accumulate a large number of microtubules within themselves, which disrupts cell functions, especially cell division, which is forced to cease at the mitotic stage.
Pharmacology
Paclitaxel is mainly used for the treatment of ovarian cancer and breast cancer. The mechanism of it includes:
1. The effects on cell microtubules/tubulin: Inhibition of microtubule depolymerization results in abnormal micro tube bundle arrangement and makes the spindle lose normal function and then induces cell death.
2. In the absence of bird triphosphate (GTP) and microtubule associated protein (MAP), it induces cells to form microtubule lack of function.
3. It significantly sensitized cancer cells to radiotherapy through blocking the cell cycle in the stage of G2 and M .
Paclitaxel is mainly metabolized through the liver and enters into the intestine with bile and then eliminated from the body by the feces (90%).
Anticancer Research
It is isolated from the bark of Taxus brevifolia generally known as pacific yew. It isprimarily used in ovarian, small, and non-small cell lung cancers and advancedbreast cancer (Shoeb 2006). It binds to tubulin but neither depolymerizes it nor interferes with its assembly (Balunas and Kinghorn 2005). Taxol targets activatorprotein 1 signaling pathways (Singh et al. 2016b).
Clinical Use
Paclitaxel is among the most active of all anticancer drugs, with significant efficacy against carcinomas of the breast, ovary, lung, head, and neck. It is combined with cisplatin in the therapy of ovarian and lung carcinomas and with doxorubicin in treating breast cancer.
Side effects
Myelosuppression is the major side effect of paclitaxel. Alopecia is common, as is reversible dose-related peripheral neuropathy. Most patients have mild numbness and tingling of the fingers and toes beginning a few days after treatment. Mild muscle and joint aching also may begin 2 or 3 days after initiation of therapy. Nausea is usually mild or absent. Severe hypersensitivity reactions may occur. Cardiovascular side effects, consisting of mild hypotension and bradycardia, have been noted in up to 25% of patients.
Toxicology
The major toxicity seen with paclitaxel is a dose-limitingmyelosuppression that normally presents as neutropenia. Thepreviously mentioned hypersensitivity reactions occur but aregreatly reduced by antihistamine pretreatment. Interactionwith the axonal microtubules such as that seen for the vincasalso occurs and leads to numbness and paresthesias (abnormaltouch sensations including burning and prickling). Theagent is also available as an albumin-bound formulation(Abraxane) to eliminate the need for the solubilizing agentsassociated with the hypersensitivity reactions. Other adverseeffects include bradycardia, which may progress to heartblock, alopecia, mucositis, and/or diarrhea. Paclitaxel producesmoderate nausea and vomiting that is short-lived.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Cytotoxics: increased risk of neutropenia with
lapatinib.
Metabolism
Paclitaxel is highly plasma protein bound (>90%) anddoes not penetrate the CNS. Metabolism involves CYPmediatedoxidation to give 6 -hydroxypaclitaxel (CYP2C8)and para hydroxylation of the phenyl group attached to the3'-position (CYP3A4). The 6α-hydroxy metabolite normallypredominates, but the para hydroxy metabolite mayoccur to a greater degree in those patients with liver diseaseor when CYP3A4 has been induced. Both metabolites areless active than the parent and do not undergo phase II conjugationreactions. Elimination occurs primarily in the feces,and the elimination half-life is 9 to 50 hours depending onthe infusion period.
storage
Store at +4°C
Precautions
1. Hermatological toxicity: the main factor in increased dosage limitations; when white blood cells are below 1500/mm3, supplement with G-CSF; when platelets are below 30000/mm3, transfuse component blood.
2. Allergic reaction: Aside from preconditions, if there are only minor symptoms such as flushed face, skin reactions, slightly increase heart rate, slightly lowered blood pressure, etc., do not stop treatment and decrease injection speed. If there are serious reactions such as hypotension, vascular edema, difficulty breathing, measles, etc., stop treatment and treat accordingly. Patients with serious allergic reactions should not use paclitaxel in the future.
3. Nervous system: Common reactions include numb toes. Approximately 4% patients, especially with high dosage, experience significant sensory and motor difficulty and decreased tendon reflex. There have been individual reports of epilepsy.
4. Cardiovascular: Transient tachycardia and hypotension are common and do not usually require attention. However, monitor closely during first hour of injection. Afterwards, only patients with serious injection difficulty require hourly check-ins.
5. Join and muscle: Approximately half of the patients will experience some joint and muscle pain within the first 2-3 days following injection, which is related to dosage, and usually subsides after a couple days. Patients who are also administered G-CSF will experience heightened muscle pain.
6. Liver and gall: As paclitaxel is mainly excreted through bile, patients with liver and gall diseases must be monitored carefully. Among thousands of cases, 8% of patients experienced increased bilirubin, 23% experienced increased alkaline phosphatase, and 18% experienced increased glutamic-oxalacetic transaminase. However, there is currently no evidence indicating that paclitaxel causes any severe liver damage.
7. Other: Digestive tract reactions are common but rarely severe, with few cases of diarrhea and mucosa infection. Slight alopecia is also common.
References
Wani et al.,J. Amer. Chem. Soc., 93,2325 (1971)
Paclitaxel Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Mesochem Technology Co.,Ltd | +8613651027935 | rachel@mesochem.com | China | 191 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Hebei baicao biology science and technology co., ltd | +86-19131911055 +8617824879454 | zhang@hbbocao.com | China | 1035 | 58 |
| Wuhan senwayer century chemical Co.,Ltd | +undefined-27-86652399 +undefined13627115097 | market02@senwayer.com | China | 874 | 58 |
| Lewoo Pharmatech(Shanghai)Co.,Ltd | +86-15800805830 +86-15800805830 | miao@lewoopharma.com.cn | China | 156 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Wuhan Haorong Biotechnology Co.,ltd | +8618565342920 | sales@chembj.net | China | 269 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7378 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 775 | 58 |
Related articles
- Paclitaxel: Medical use, mechanism, side effects
- Paclitaxel is a chemotherapeutic agent marketed under the brand name Taxol among others. Used as a treatment for various cance....
- May 11,2023
- The uses and Pharmacologic action of Paclitaxel
- Paclitaxel is a chemotherapeutic agent marketed under the brand name Taxol among others. Used as a treatment for various cance....
- Aug 22,2019
Related Qustion
- Q:What is the mechanism that causes paclitaxel resistance?
- A:Mechanisms of resistance to paclitaxel have also been extensively studied, such as overexpression of ABC transporters, altered....
- Dec 1,2023
View Lastest Price from Paclitaxel manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-15 | Paclitaxel
33069-62-4
|
US $0.00 / kg | 1kg | 99% | 20tons | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-04-12 | Paclitaxel
33069-62-4
|
US $0.00 / kg | 1kg | 99% | 2000ton | Shaanxi Haibo Biotechnology Co., Ltd | |
 |
2024-04-10 | Paclitaxel
33069-62-4
|
US $120.00-25.00 / g/Bag | 1g/Bag | 99% up, USP34, GMP, DMF | 20tons | Sinoway Industrial co., ltd. |
-

- Paclitaxel
33069-62-4
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Paclitaxel
33069-62-4
- US $0.00 / kg
- 99%
- Shaanxi Haibo Biotechnology Co., Ltd
-

- Paclitaxel
33069-62-4
- US $120.00-25.00 / g/Bag
- 99% up, USP34, GMP, DMF
- Sinoway Industrial co., ltd.