Cesium carbonate
- CAS No.
- 534-17-8
- Chemical Name:
- Cesium carbonate
- Synonyms
- CAESIUM CARBONATE;Caesium carbonate 99.9%;LT-E002;60 - 80 Mesh;JACS-534-17-8;cesiumcabonate;CESIUM CARBONATE;dicesiumcarbonate;CesiumCarbonateGr;TCesium carbonate
- CBNumber:
- CB3274457
- Molecular Formula:
- CCs2O3
- Molecular Weight:
- 325.82
- MDL Number:
- MFCD00010957
- MOL File:
- 534-17-8.mol
- MSDS File:
- SDS
| Melting point | 610 °C (dec.) (lit.) |
|---|---|
| Density | 4.072 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | soluble in Water |
| form | Powder/Granules |
| color | White |
| Specific Gravity | 4.072 |
| Water Solubility | 261 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,2010 |
| BRN | 4546405 |
| Stability | Stable. Very deliquescent. Incompatible with strong oxidizing agents, strong acids. |
| InChIKey | FJDQFPXHSGXQBY-UHFFFAOYSA-L |
| LogP | 0 at 20℃ |
| CAS DataBase Reference | 534-17-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | QQI20A14P4 |
| EPA Substance Registry System | Carbonic acid, dicesium salt (534-17-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H318-H361f-H373 | |||||||||
| Precautionary statements | P201-P202-P260-P280-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 68-36/37/38 | |||||||||
| Safety Statements | 22-24/25-36/37/39-26-27 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | FK9400000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28369918 | |||||||||
| NFPA 704 |
|
Cesium carbonate price More Price(102)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 20960 | Cesium carbonate purum p.a., ≥98.0% (T) | 534-17-8 | 10g | $33 | 2024-03-01 | Buy |
| Sigma-Aldrich | 20960 | Cesium carbonate purum p.a., ≥98.0% (T) | 534-17-8 | 50g | $92.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 20960 | Cesium carbonate purum p.a., ≥98.0% (T) | 534-17-8 | 250g | $198 | 2024-03-01 | Buy |
| Sigma-Aldrich | 202126 | Cesium carbonate 99.9% trace metals basis | 534-17-8 | 1kg | $666 | 2024-03-01 | Buy |
| Sigma-Aldrich | 202126 | Cesium carbonate 99.9% trace metals basis | 534-17-8 | 2.5kg | $1610 | 2024-03-01 | Buy |
Cesium carbonate Chemical Properties,Uses,Production
Description
Cesium carbonate is an inorganic compound. It is a white solid under normal temperature and pressure. It is easily soluble in water and quickly absorbs moisture when placed in the air. Cesium carbonate aqueous solution is strongly alkaline and can react with acid to produce corresponding cesium salt and water, and release carbon dioxide. Cesium carbonate is easy to transform, can be used as a precursor of other cesium salts, and is a widely used cesium salt. 
Uses
Cesium carbonate can be used as a modified layer between the active layer of the solar cell and the Al electrode to improve the conversion efficiency of the device, generate Al-O-Cs compounds, reduce the series resistance of the battery, and improve the short-circuit current and photoelectric conversion efficiency of the solar cell, and Due to the compactness of the cesium carbonate layer itself, the stability of the device has also been significantly improved. The strong metallic n-type heavy doping effect of cesium in cesium carbonate, the Al/CsCO cathode modified with cesium carbonate nano-interface can greatly increase the injection of electrons, and significantly improve the performance of the inverted top-emitting structure OLED device. Cesium carbonate is also used as a catalyst for the synthesis of alkyl aryl ethers.
Preparation
The method of preparing cesium carbonate using cesium alum as a raw material :1. To recrystallize cesium alum, add cesium alum in deionized water and heat to dissolve, after dissolving, cool to crystallize and filter, and obtain refined cesium alum after filtering;2. Transformation, add refined cesium alum in deionized water and heat to dissolve, obtain refined cesium alum solution after dissolving, slowly drip lime milk into refined cesium alum solution, and obtain cesium sulfate net solution after solid-liquid separation;3. Causticizing, the barium hydroxide solution is added to the cesium sulfate clean liquid in 2 to 3 times, and the barium sulfate solid and the causticizing liquid are obtained by centrifugal separation;4. Carbonization, concentration and evaporation, pass CO into the causticizing solution for carbonization, carbonize until the pH of the carbonization solution is 7 to 10, stand still, concentrate the carbonization supernatant after standing, and obtain a concentrated solution after cooling;5. Once fine filtration and drying, the concentrated liquid is filtered with a polymer filter device, the filtered filtrate is concentrated and crystallized and dried at low temperature to obtain a low-temperature material;6. High temperature decomposition, after high temperature decomposition of low temperature material, high temperature material is obtained;7. Secondary fine filtration, the high temperature material is dissolved in deionized water, and then filtered with a polymer filter device, and the filtered liquid is obtained after filtration;8. The clean liquid is concentrated and dried, and the filtered clean liquid is concentrated and dried to obtain cesium carbonate.
Description
Cesium carbonate is a basic carbonate and is used in organic synthesis as a mild inorganic base. It can be used in C, N, O alkylation and arylation reactions. Cesium carbonate not only promotes the carbonylation of alcohols and carbamination of amines but also inhibits side reactions common in other processes. It is also used in six-membered annulation, intramolecular and intermolecular cyclizations, Suzuki coupling, aza-Henry, nucleophilic substitution, cross-coupling and different cycloaddition reactions.
Chemical Properties
Colourless crystals or white powder , easily soluble in water, quickly absorbs moisture when placed in the air. The aqueous solution of cesium carbonate is strongly alkaline and can react with acid to produce the corresponding cesium salt and water, and release carbon dioxide.
Uses
Cesium carbonate is a versatile reagent for organic synthesis. This inorganic base has been employed in numerous organic applications ranging from N-protection of amino acids to a base in either the Horner- Wadsworth-Emmons reaction or in Suzuki couplings. It has also found use as a catalyst for ethylene oxide polymerization, in coating for spatter-free welding of steel in CO2, as a functional interlayer in photovoltaic devices and in oxide cathodes. Cesium carbonate is also used in the beer-brewing industry to make the "head" of beer foamier.
Application
Cesium carbonate is widely utilized as a precursor for other cesium compounds. It acts as a base in sensitive organic reactions. It can be used as a base in C-C and C-N cross-coupling reactions such as Suzuki?Miyaura, Heck, and Buchwald-Hartwig amination reactions. It finds use in solar cells as it increases the power conversion efficiency of cells through the transfer of electrons. It is also used in the production of special optical glasses, petroleum catalytic additives, special ceramics and in the sulfuric acid industry. It is useful in the N-alkylation (of sulfonamides, beta-lactams, indoles, heterocycles and several sensitive nitrogen compounds), carbamination of amines, carbonylation of alcohols and aerobic oxidation of alcohols into carbonyl compounds without polymeric by products. Promotes the efficient O-alkylation of alcohols to form mixed alkyl carbonates.
Preparation
cesium carbonate can be prepared by thermal decomposition of caesium oxalate. Upon heating, caesium oxalate is converted to caesium carbonate with emission of carbon monoxide.
Cs2C2O4 → Cs2CO3 + CO
It can also be synthesized by reacting caesium hydroxide with carbon dioxide.
2 CsOH + CO2 → Cs2CO3 + H2O
Reactions
Many of the properties of cesium carbonate are due to the softness of the cesium cation. This softness makes cesium carbonate rather soluble in organic solvents such as alcohols, DMF and Et2O. This has rendered cesium carbonate useful in palladium chemistry, which is often carried out in non-aqueous media where insolubility of inorganic bases can limit reactivity. Cs2CO3 has, for example, been used with good results in Heck, Suzuki and Sonogashira reactions. Cesium carbonate has also received much attention for its use in O-alkylations, particularly of phenols.It has been postulated that O-alkylations of phenols using Cs2CO3 in non-aqueous solvents occurs via the ‘naked’ phenolate anion, which behaves as a strong nucleophile. Therefore, this methodology can even be applied to secondary halides, minimizing the usual unwanted side reactions such as elimination and decomposition.
Reactions
Inorganic bases include LiOt
Bu, NaOt
Bu, NaOAc, LiOH, NaOH, KOH, Na2CO3, K2CO3, Cs2CO3, ect. Organic bases include DBU and Et3N, etc. Cesium carbonate has a similar basicity with DBU. Due to the poor solubility of most inorganic bases in organic solvents and the aggregation between metal ions and solvent molecules, which may cause some side-effect to the basicity of bases. The solubility of metal carbonate in dipole aprotic solvents is shown below.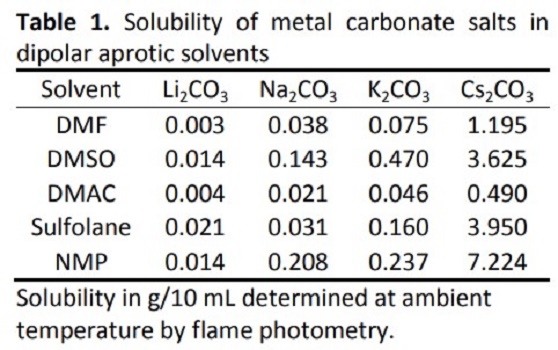
In the transition metal catalytic organic reactions, different bases were chosen in the
palladium catalyzed reaction system, leading to a
sharp change in reaction yields. In the direct
carboxylation of indole mediated by LiOt
Bu. Cs2CO3 as a strong base enhanced reaction yield dramatically, while K2CO3 as a weak base can only generate very little carboxyl products. The most
commonly used bases in Suzuki reactions are
Na2CO3, Cs2CO3, potassium acetate, potassium
phosphate etc[1]. The reactive order of alkali
carbonates are as follows: Cs2CO3 > K2CO3 >
Na2CO3 > Li2CO3. Caesium carbonate facilitates the N-alkylation of compounds such as sulfonamides, amines, β-lactams, indoles, heterocyclic compounds, N-substituted aromatic imides, phthalimides, and other similar compounds.
General Description
Cesium carbonate is a powerful inorganic base widely used in organic synthesis. It is a potential chemoselective catalyst for the reduction of aldehydes and ketones to alcohols. It is employed as base for the Heck coupling reaction of 4-trifluoromethyl-1-chlorobenzene and aryl chlorides.
Hazard
Cesium carbonate is a danger compound.
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
H361: Suspected of damaging fertility or the unborn child
H373: May cause damage to organs through prolonged or repeated exposure
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes. See also CESIUM.
Purification Methods
Crystallise it from ethanol (10mL/g) by partial evaporation. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 988 1963.]
References
[1] Guo L, et al. Applications of Bases in Transition Metal Catalyzed Reactions Zhao Yuan. Chemistry - A European Journal, 2018; 24: 7794-7809.
Cesium carbonate Preparation Products And Raw materials
Raw materials
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Chemicalsolution | +86-13661650151 | syuzwx@126.com | China | 4 | 58 |
| CHANGZHOU HARVECHEM LTD | +86-0519-88784888 +8613906120163 | inquiry@harvechem.com | China | 245 | 58 |
| Shanghai Huaran Industrial Co.,Ltd | 17749774353 | 1715438216@qq.com | China | 52 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Hunan aslsen technology co.,ltd | +8615308460054 | aslsc@aslsen.com | China | 124 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6711 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7377 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 951 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 343 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
Related articles
- Cesium Carbonate Promoted Direct Amidation of Unactivated Esters with Amino Alcohol Derivatives
- Cesium carbonate is a white hygroscopic powder that is readily soluble in water.
- Apr 17,2024
- Application and Property of Cesium carbonate
- Cesium carbonate is a basic carbonate used in organic synthesis as a mild inorganic base.
- Sep 8,2023
- The review of Cesium carbonate
- Cesium carbonate has also found much use in solid supported synthesis, where solubility can be of importance.
- Jan 28,2022
View Lastest Price from Cesium carbonate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-19 | Cesium carbonate
534-17-8
|
US $50.00 / kg | 1kg | 99.10% | 5000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-15 | Cesium Carbonate
534-17-8
|
US $192.00-7000.00 / KG | 1KG | 97% | 1 ton | Zhengzhou Alfa Chemical Co.,Ltd | |
 |
2024-04-12 | Cesium carbonate
534-17-8
|
US $30.00 / kg | 1kg | 98% | 2000kg | hebei hongtan Biotechnology Co., Ltd |
-

- Cesium carbonate
534-17-8
- US $50.00 / kg
- 99.10%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- Cesium Carbonate
534-17-8
- US $192.00-7000.00 / KG
- 97%
- Zhengzhou Alfa Chemical Co.,Ltd
-

- Cesium carbonate
534-17-8
- US $30.00 / kg
- 98%
- hebei hongtan Biotechnology Co., Ltd