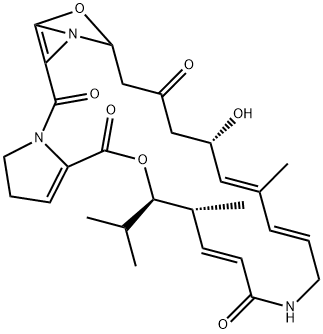
VIRGINIAMYCIN M1
- CAS No.
- 21411-53-0
- Chemical Name:
- VIRGINIAMYCIN M1
- Synonyms
- Streptogramin A;C11299;PA 114A;Factor M;NSC 87432;NSC 244426;MIKAMYCIN A;Vernamycin A;STAPHYLOMYCIN;AVirginiaMyci
- CBNumber:
- CB3294594
- Molecular Formula:
- C28H35N3O7
- Molecular Weight:
- 525.59
- MDL Number:
- MFCD00869411
- MOL File:
- 21411-53-0.mol
- MSDS File:
- SDS
| Melting point | 165-167℃ |
|---|---|
| alpha | D20 -218° ( c = 0.34 in ethanol) |
| Boiling point | 825.2±65.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | chloroform/methanol: soluble10mg/mL |
| form | powder |
| pka | 13.18±0.70(Predicted) |
| color | yellow to tan |
| Stability | Light Sensitive |
| FDA UNII | 8W4UOL59AZ |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P271-P280 |
| WGK Germany | 3 |
| HS Code | 29419090 |
VIRGINIAMYCIN M1 price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | V2753 | Virginiamycin M1 ~95% | 21411-53-0 | 5mg | $459.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | V2753 | Virginiamycin M1 ~95% | 21411-53-0 | 10mg | $1030 | 2024-03-01 | Buy |
| Cayman Chemical | 9002172 | Virginiamycin M1 ≥95% | 21411-53-0 | 5mg | $347 | 2024-03-01 | Buy |
| Cayman Chemical | 9002172 | Virginiamycin M1 ≥95% | 21411-53-0 | 25mg | $1210 | 2024-03-01 | Buy |
| TRC | V673810 | VirginiamycinM1(~90%) | 21411-53-0 | 100mg | $1450 | 2021-12-16 | Buy |
VIRGINIAMYCIN M1 Chemical Properties,Uses,Production
Chemical Properties
Light yellow powder
Uses
Macrolactone antibiotic. Antibacterial; growth promotant.
Uses
Ostreogrycin A (virginiamycin M1, streptogramin A) is the major component of the virginiamycin complex. In the 1950s this complex was independently discovered so many times that the literature became highly confusing. Ostreogrycin A is a macrocyclic lactone antibiotic that acts synergistically with the structurally unrelated cyclic depsipeptides, virginiamycin B (ostreogrycin B, streptogramin B) and virginiamycin S, to inhibit peptide elongation. This is achieved by blocking formation of a peptide bond between the growing peptide chain (peptidyl-tRNA) linked to the 50S ribosome and aminoacyl-tRNA. Ostreogrycin A is highly active against Gram positive bacteria, particularly MRSA.
Uses
Ostreogrycin A (virginiamycin M1, streptogramin A) is the major component of the virginamycin complex. In the 1950s this complex was independently discovered so many times that the literature became highly confusing. Ostreogrycin A is a macrocyclic lactone antibiotic that acts synergistically with the structurally unrelated cyclic depsipeptides, virginiamycin B (ostreogrycin B, streptogramin B) and virginiamycin S, to inhibit peptide elongation. This is achieved by blocking formation of a peptide bond between the growing peptide chain (peptidyl-tRNA) linked to the 50S ribosome and aminoacyl-tRNA. Ostreogrycin A is highly active against Gram positive bacteria, particularly MRSA.
Definition
ChEBI: A macrolide that is (together with pristinamycin IA) a component of pristinamycin, an oral streptogramin antibiotic produced by Streptomyces pristinaespiralis. Pristinamycin exhibits bactericidal activity against Gram positive organisms includ ng methicillin-resistant Staphylococcus aureus.
Biological Activity
virginiamycin m1 is a macrolide antibiotic that reversibly inhibits protein synthesis [1][2][3].virginiamycin complex contains two antibiotics, virginiamycin m1 and virginiamycin s1. streptogramins are divided into class a and class b based on their structures. virginiamycin m1, also known as streptogramin a, is a member of the streptogramin a group of antibiotics, which bind the 50s ribosomal subunit at the peptidyl transferase center to inhibit initiation and translocation. they show good bactericidal activity against methicillin-resistant s. aureus (mrsa), although resistance in mrsa is conferred by the cfr gene. virginiamycin m1 has activity against gram-positive and in select cases gram-negative bacteria. combination of group a and b streptogramins exhibit bactericidal activity [1]. virginiamycin m1 acted synergistically with virginiamycin s1 to irreversibly inhibit protein synthesis in bacteria. in cell-free systems, virginiamycin m1 and virginiamycin s1 bound to the large ribosomal subunit, and the affinity of ribosomes for vs is increased by vm [2][3].
Contact allergens
Pristinamycin is a systemic antibiotic of the synergistins/ streptogramins class, composed of two subunits: pristinamycin IA and pristinamycin IIA. It induces several types of drug reactions such as maculo-papular exanthema, systemic dermatitis, or acute generalized exanthematous pustulosis. Some patients have been previously skin-sensitized by virginiamycin. Crossreactivity is expected to virginiamycin and to the associated dalfopristin and quinupristin.
Contact allergens
Like the other streptogramin, pristinamycin, virginiamycin is made of two subunits, virginiamycin S1 and virginiamycin M1. Dermatitis was quite common in people using the formerly available topical virginiamycin. Occupational dermatitis was observed in the pharmaceutical industry, in breeders, and in a surgeon who used topical virginiamycin on postoperative wounds (personal observation).
References
[1]. fair rj, tor y. antibiotics and bacterial resistance in the 21st century. perspect medicin chem. 2014 aug 28;6:25-64.
[2]. kehrenberg c, cuny c, strommenger b, et al. methicillin-resistant and -susceptible staphylococcus aureus strains of clonal lineages st398 and st9 from swine carry the multidrug resistance gene cfr. antimicrob agents chemother. 2009 feb;53(2):779-81.
[3]. parfait r, cocito c. lasting damage to bacterial ribosomes by reversibly bound virginiamycin m. proc natl acad sci u s a. 1980 sep;77(9):5492-6.
VIRGINIAMYCIN M1 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| BOC Sciences | +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +8619164747840 | 13119157289@163.com | China | 2971 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Chemsky(shanghai)International Co.,Ltd. | 021-50135380 | shchemsky@sina.com | China | 32344 | 50 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12338 | 58 |
| 9ding chemical ( Shanghai) Limited | 4009209199 | sales@9dingchem.com | China | 22519 | 55 |
View Lastest Price from VIRGINIAMYCIN M1 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2019-12-26 | VIRGINIAMYCIN M1
21411-53-0
|
US $1.00 / g | 1g | ≥98% | g/kg/Ton | Career Henan Chemical Co |
-

- VIRGINIAMYCIN M1
21411-53-0
- US $1.00 / g
- ≥98%
- Career Henan Chemical Co
21411-53-0(VIRGINIAMYCIN M1)Related Search:
1of4