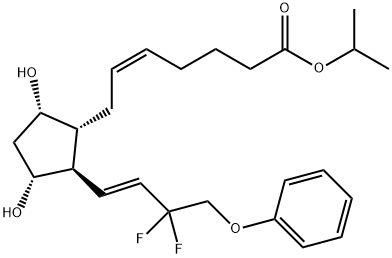
Tafluprost
- CAS No.
- 209860-87-7
- Chemical Name:
- Tafluprost
- Synonyms
- aflupros;TafL;MK2452;uprost;AFP-168;TAFLUPROST;Tafluprosttrade;Tafluprost USP/EP/BP;209860-87-7 Tafluprost;He has fluprostaglandin
- CBNumber:
- CB3401660
- Molecular Formula:
- C25H34F2O5
- Molecular Weight:
- 452.53
- MDL Number:
- MFCD08062150
- MOL File:
- 209860-87-7.mol
- MSDS File:
- SDS
| Boiling point | 552.9±50.0 °C(Predicted) |
|---|---|
| Density | 1.186 |
| storage temp. | 2-8°C |
| solubility | soluble in Chloroform, DMSO, Ethyl Acetate |
| form | Clear, colorless to slightly yellow oil |
| pka | 14.48±0.70(Predicted) |
| FDA UNII | 1O6WQ6T7G3 |
| ATC code | S01EE05 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H372-H360-H319-H413-H370-H302 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P260-P264-P270-P307+P311-P321-P405-P501-P264-P270-P301+P312-P330-P501-P260-P264-P270-P314-P501 | |||||||||
| NFPA 704 |
|
Tafluprost price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 10005440 | Tafluprost ≥98% | 209860-87-7 | 1mg | $112 | 2024-03-01 | Buy |
| Cayman Chemical | 10005440 | Tafluprost ≥98% | 209860-87-7 | 5mg | $499 | 2024-03-01 | Buy |
| Cayman Chemical | 10005440 | Tafluprost ≥98% | 209860-87-7 | 10mg | $884 | 2024-03-01 | Buy |
| Cayman Chemical | 10005440 | Tafluprost ≥98% | 209860-87-7 | 25mg | $1381 | 2024-03-01 | Buy |
| Tocris | 6427 | Tafluprost ≥95%(HPLC) | 209860-87-7 | 5 | $245 | 2021-12-16 | Buy |
Tafluprost Chemical Properties,Uses,Production
Description
Glaucoma is second only to cataracts as a causative factor of blindness.
By 2010, it is estimated that approximately 60 million people worldwide
will be afflicted by glaucoma, so effective treatments should garner a large market.
PG analogs have been widely used for lowering IOP by increasing uveoscleral outflow through agonism of the prostanoid FP
receptor, and currently marketed versions include latanoprost, unoprostone isopropyl ester, bimatoprost, and travoprost.
Compared to the carboxylic acid of latanaprost
(Ki=4.7 nM), the carboxylic acid of tafluprost displayed a 10-fold greater
affinity for the prostanoid FP receptor (Ki=0.4 nM). The synthesis of
tafluprost begins with a Wittig condensation of the protected bicyclic
lactone carbaldehyde with a dimethyl phosphonate ketone derivative.
Compared to the carboxylic acid of latanaprost (Ki 4.7 nM), the carboxylic acid of tafluprost displayed a 10-fold greater affinity for the prostanoid FP receptor (Ki 0.4 nM). The synthesis of tafluprost begins with a Wittig condensation of the protected bicyclic lactone carbaldehyde with a dimethyl phosphonate ketone derivative. The bottom appendage is then completed by the fluorination of the ketone with morpholino-sulfur trifluoride. Hydrolysis of the benzoate ester protecting group liberates the hydroxy group, and reduction of the lactone is accomplished with aluminum hydride to generate the lactol. Condensation of this intermediate with the phosphonium salt of the acid side chain generates the free acid, or active ingredient, which is subsequently esterified with 2-iodopropane in the presence of DBU.
.
Originator
Santen/Asahi Glass (Japan)
Uses
Tafluprost is a novel prostanoid used in the treatment of glaucoma and is the first prostanoid to be released in a preservative free-formula.
Definition
ChEBI: Tafluprost is a prostaglandin Falpha that is prostaglandin F2alpha in which the carboxylic acid function has been converted to the corresponding isopropyl ester and the 3-hydroxy-1-octenyl side-chain is substituted by 3,3-difluoro-4-phenoxybut-1-enyl. Used for treatment of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. It has a role as a prostaglandin receptor agonist. It is a prostaglandins Falpha, an organofluorine compound and an isopropyl ester. It is functionally related to a prostaglandin F2alpha.
brand name
Taflotan
Synthesis
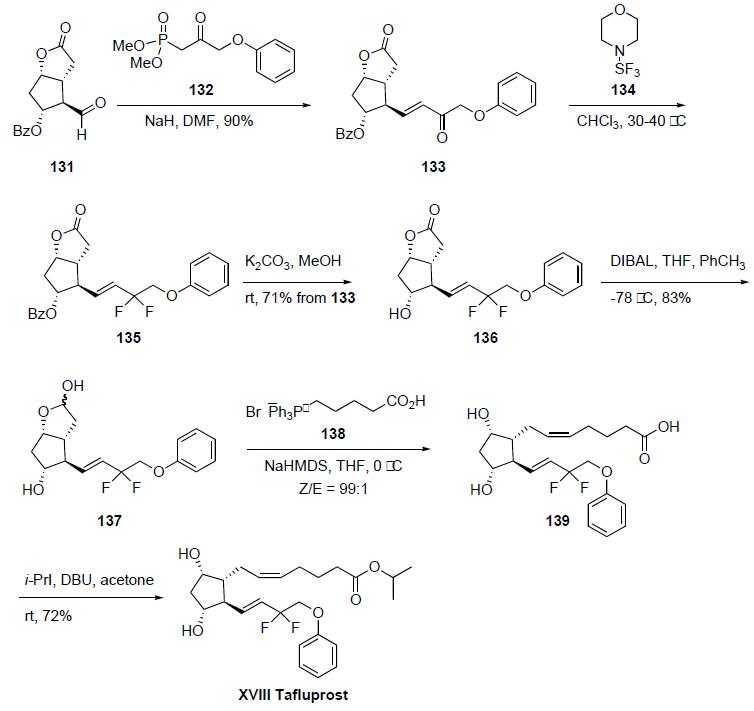
The synthesis was initiated from the Corey aldehyde 131. Horner-Emmons condensation of the bicyclic carbaldehyde 131 with the dimethyl phosphonate 132 using NaH in DMF gave enone 133 in 90% yield. Fluorination of the enone 133 was accomplished upon reaction with morpholino-sulfur trifluoride (134) in chloroform to yield the corresponding difluorinated compound 135. Hydrolysis of the benzoate ester group of 135 with K2CO3 in methanol at room temperature afforded alcohol 136 in 71% yield from 133. Reduction of the lactone group of 136 with diisobutyl aluminum hydride (DIBAL) in THF/toluene gave lactol 137 in 83% yield. Lactol 137 was condensed with the phosphonium ylide prepared by deprotonation of phosphonium salt 138 with sodium bis(trimethylsilyl)amide (NaHMDS) in THF/toluene to give the prostaglandin F2-alpha derivative 139 in excellent Z/E selectivity (99:1). The synthesis was completed by esterification of compound 139 with isopropyl iodide and DBU in acetone to provide tafluprost (XVIII) in 72% yield.
Mode of action
Tafluprost(209860-87-7) is a Prostaglandin Analog. The mechanism of action of tafluprost is as a Prostaglandin Receptor Agonist. It is believed that prostanoid FP-receptor agonists such as tafluprost reduce IOP by increasing the uveoscleral outflow of aqueous humor. There is some evidence that tafluprost may lower IOP by interaction with the EP3 receptor.
DB08819
Tafluprost Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9353 | 55 |
| Shanghai finete Pharmaceutical Co., Ltd. | +86-18221039705 | CHINA | 139 | 55 | |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | 18957127338 | stella@zetchem.com | China | 2141 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
View Lastest Price from Tafluprost manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-10 | Tafluprost
209860-87-7
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-07 | Tafluprost
209860-87-7
|
US $0.00-0.00 / g | 1g | 99.9% | 20tons | airuikechemical co., ltd. | |
 |
2023-03-12 | Tafluprost
209860-87-7
|
US $0.00 / kg | 1kg | 98.0%min | 100kg | LY Global chemicals co.,ltd . |
-

- Tafluprost
209860-87-7
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- Tafluprost
209860-87-7
- US $0.00-0.00 / g
- 99.9%
- airuikechemical co., ltd.
-

- Tafluprost
209860-87-7
- US $0.00 / kg
- 98.0%min
- LY Global chemicals co.,ltd .
209860-87-7(Tafluprost)Related Search:
1of4