Aluminum oxide
- CAS No.
- 11092-32-3
- Chemical Name:
- Aluminum oxide
- Synonyms
- RUBY;AlO2;ALUNDUM;ALUMINA;CORUNDUM;CORUNDUM;SAPPHIRE;CORUNDUM;ALUMINA B;ALUMINA C
- CBNumber:
- CB3490231
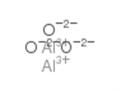
- Molecular Formula:
- AlO2
- Molecular Weight:
- 58.98
- MDL Number:
- MFCD00003424
- MOL File:
- 11092-32-3.mol
- MSDS File:
- SDS
| Melting point | 2040 °C(lit.) |
|---|---|
| Boiling point | 2977 °C |
| Density | 1.06 g/mL at 25 °C |
| form | powder |
| Water Solubility | Insoluble in water. |
| Exposure limits |
ACGIH: TWA 1 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 |
| Dielectric constant | 4.5(Ambient) |
| CAS DataBase Reference | 11092-32-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| ATC code | D10AX04 |
Aluminum oxide price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Alfa Aesar | 036649 | Aluminum oxide, single crystal | 11092-32-3 | 1pc | $75.7 | 2021-12-16 | Buy |
| Alfa Aesar | 036649 | Aluminum oxide, single crystal | 11092-32-3 | 5pc | $276 | 2021-12-16 | Buy |
| Alfa Aesar | 044849 | Aluminum oxide, catalyst support, low silica | 11092-32-3 | 5kg | $547 | 2021-12-16 | Buy |
| Alfa Aesar | 044849 | Aluminum oxide, catalyst support, low silica | 11092-32-3 | 1kg | $140 | 2021-12-16 | Buy |
Aluminum oxide Chemical Properties,Uses,Production
Characteristics
Aluminum Oxide (Alumina) is the most widely used oxide, chiefly because it is plentiful, relatively low in cost, and equal to or better than most oxides in mechanical properties. Density can be varied over a wide range, as can purity — down to about 90% alumina — to meet specific application requirements. Alumina ceramics are the hardest, strongest, and stiffest of the oxides. They are also outstanding in electrical resistivity, dielectric strength, are resistant to a wide variety of chemicals, and are unaffected by air, water vapor, and sulfurous atmospheres. However, with a melting point of only 2039°C, they are relatively low in refractoriness, and at 1371°C retain only about 10% of room-temperature strength. In addition to its wide use as electrical insulators and its chemical and aerospace applications, the high hardness and close dimensional tolerance capability of alumina make this ceramic suitable for such abrasion-resistant parts as textile guides, pump plungers, chute linings, discharge orifices, dies, and bearings.
Uses
Aluminum oxide is used for the separation of both inorganic anions and acidic organic molecules such as acidic amino acids, aromatic acids and carboxylic acids. It is essential in protein extraction as a component in the preparation of tissues. It is also used as a grinding or blending agent. Aluminum oxide is a source of aluminum in reactions, an abrasive agent, and as a refractory material. Also Aluminum oxide can act as a catalyst in a variety of reactions such as the claus process and in the dehydration of alcohols to alkenes. In single crystals of aluminum oxide intrinsic diffusion occurs in a high temperature region.
Definition
A mineral form of aluminiumoxide, Al2O3. It crystallizesin the trigonal system and occurs aswell-developed hexagonal crystals. Itis colourless and transparent whenpure but the presence of other elementsgives rise to a variety ofcolours. Ruby is a red variety containingchromium; sapphire is ablue variety containing iron andtitanium. Corundum occurs as arock-forming mineral in both metamorphicand igneous rocks. It ischemically resistant to weatheringprocesses and so also occurs in alluvial(placer) deposits. The secondhardest mineral after diamond (it hasa hardness of 9 on the Mohs’ scale), itis used as an abrasive.
Preparation
Pure alumina, needed to produce aluminum by the Hall process, is made by the Bayer process. The starting material is bauxite (Al2O3 ? nH2O). The ore contains impurities, such as, SiO2, Fe2O3, TiO2, and Na2O. Most impurities are removed following treatment with caustic soda solution. Bauxite is dissolved in NaOH solution. Silica, iron oxides and other impurities are filtered out of the solution. CO2 is then bubbled through this solution. This precipitates out hydrated alumina, which is heated to remove water and produce Al2O3. These impurities are removed. Calcinations of bauxite produce alumina of abrasive and refractory grades. Activated aluminas of amorphous type, as well as the transition aluminas of γ, η, χ, and ρ forms, are obtained from various aluminum hydroxides, such as, α- and β-trihydrates, α-monohydrate and alumina gel. Such chemicals are obtained from bauxite by the Bayer process also.
Hazard
Chronic inhalation of Al2O3 dusts may cause lung damage.
Agricultural Uses
Alumina is a white or colorless oxide occurring in two
forms, a-alumina and γ-alumina. The γ-alumina turns
into a stable a form on heating. Naturally occurring
alumina is called corundum or emery.
The gemstones ruby and sapphire are aluminum
oxides colored by minute traces of chromium and cobalt,
respectively. The highly protective film of oxide formed
on the surface of aluminum is yet another structural
variation, a defective form of rock salt.
Pure aluminum oxide is obtained by dissolving
bauxite ore in sodium hydroxide solution to eliminate
insoluble impurities. Seeding the solution with material
from a previous batch precipitates the hydrated oxide,
which on further heating gives γ-alumina at 500 to 800°C
and pure a-alumina at 1150 to 1200°C. The latter is one
of the hardest materials known. It is used widely as an
abrasive substance in both its natural and synthetic forms.
Its refractory nature makes alumina bricks an ideal
material for furnace linings and high temperature
cements.
Alumina occurs in phosphate rocks along with iron
and other impurities in small percentages. Alumina and
iron in phosphate rock make the superphosphate moist
and sticky. The maximum acceptable alumina and iron in
the rock for farming is 3 to 4 %
Materials Uses
Fused aluminum oxide was the second synthetic abrasive to be developed. Synthetic aluminum oxide (alumina) is made as a white powder and can be somewhat harder than corundum (natural alumina) because of its purity. However, corundum has a Mohs hardness of approximately 9 (on a scale of 1 to 10. Alumina can be processed with different properties by slight alteration of the reactants in the manufacturing process. Several grain sizes of alumina are available, and alumina has largely replaced emery for several abrasive uses. Aluminum oxide is widely used to make bonded abrasives, coated abrasives, and air-propelled grit abrasives for dental applications.
Sintered aluminum oxide is used to make white stones, which are popular for adjusting dental enamel and finishing metal alloys, resin-based composites, and ceramic materials.
Pink and ruby variations of aluminum oxide abrasives are made by adding chromium compounds to the original melt. These variations are sold in a vitreous-bonded form as noncontaminating mounted stones for the preparation of metal– ceramic alloys to receive porcelain. Remnants of these abrasives and other debris should be removed from the surface of metals used for metal–ceramic bonding so as not to prevent optimal bonding of porcelain to the metal alloy. A review by Yamamoto (see Selected Reading) suggests that carbide burs are the most effective instruments for finishing this type of alloy because they do not contaminate the metal surface with entrapped abrasive particles.
CORUNDUM: This mineral form of aluminum oxide is usually white. Its physical properties are inferior to those of manufactured alpha (α) aluminum oxide (Al2O3), which has largely replaced corundum in dental applications. Corundum is used primarily for grinding metal alloys and is available as a bonded abrasive in several shapes. It is most commonly used in an instrument known as a white stone.
Aluminum oxide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Chemson Industrial (Shanghai) Co., Ltd. | 86-21-65208861- 8007 | sales1@chemson.com.cn | CHINA | 117 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9309 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
Related articles
- The properties and uses of Corundum
- Corundum is a rock-forming mineral that is found in igneous, metamorphic, and sedimentary rocks.
- Nov 19,2019
View Lastest Price from Aluminum oxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-02-01 | aluminum oxide
11092-32-3
|
US $1.00 / KG | 1KG | Min98% HPLC | G/KG/TON | Career Henan Chemical Co | |
 |
2018-04-25 | Alumina; Aluminum Oxide
11092-32-3
|
US $22.60 / KG | 25KG | 99.999%; 99.99%; 99.9% | 1000tons | Chemson Industrial (Shanghai) Co., Ltd. |
-

- aluminum oxide
11092-32-3
- US $1.00 / KG
- Min98% HPLC
- Career Henan Chemical Co
-

- Alumina; Aluminum Oxide
11092-32-3
- US $22.60 / KG
- 99.999%; 99.99%; 99.9%
- Chemson Industrial (Shanghai) Co., Ltd.
11092-32-3(Aluminum oxide)Related Search:
1of4





