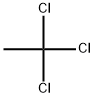
1,1,1-Trichloroethane
- CAS No.
- 71-55-6
- Chemical Name:
- 1,1,1-Trichloroethane
- Synonyms
- cf2;Trichloroethane;CH3CCl3;TRICHLORETHANE;METHYLCHLOROFORM;1,1,1-TCE;Chlorothene NU;1,1,1-Trichlorethan;1,1,1- threeethyl chloride;CF 2
- CBNumber:
- CB3701849
- Molecular Formula:
- C2H3Cl3
- Molecular Weight:
- 133.4
- MDL Number:
- MFCD00000806
- MOL File:
- 71-55-6.mol
| Melting point | −35 °C(lit.) |
|---|---|
| Boiling point | 74-76 °C(lit.) |
| Density | 1.336 g/mL at 20 °C(lit.) |
| vapor density | 4.6 (vs air) |
| vapor pressure | 100 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 11 °C |
| storage temp. | 0-6°C |
| solubility | Sparingly soluble in ethyl alcohol; freely soluble in carbon disulfide, benzene, ethyl ether, methanol, carbon tetrachloride (U.S. EPA, 1985), and many other organic solvents. |
| form | Fluid |
| Water Solubility | 1.4 g/L (20 ºc) |
| Merck | 13,9710 |
| Henry's Law Constant | 2.77 at 40 °C, 4.27 at 50 °C, 6.31 at 60 °C, 7.91 at 70 °C, 8.98 at 80 °C (headspace-GC, Vane et al., 2001) |
| Dielectric constant | 7.9(19℃) |
| Exposure limits | TLV-TWA 350 ppm (~1900 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 450 ppm (~2450 mg/m3) (ACGIH); IDLH 1000 ppm (NIOSH). |
| InChIKey | UOCLXMDMGBRAIB-UHFFFAOYSA-N |
| EPA Primary Drinking Water Standard | MCL:0.2,MCLG:0.2 |
| LogP | 2.490 |
| CAS DataBase Reference | 71-55-6(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | 1,1,1-TRICHLOROETHANE |
| FDA 21 CFR | 175.105; 177.1650 |
| EWG's Food Scores | 4-5 |
| FDA UNII | 113C650IR1 |
| IARC | 3 (Vol. 20, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Ethane, 1,1,1-trichloro-(71-55-6) |
| EPA Substance Registry System | 1,1,1-Trichloroethane (71-55-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H420 | |||||||||
| Precautionary statements | P502 | |||||||||
| Hazard Codes | Xn,N,T,F | |||||||||
| Risk Statements | 20-59-66-40-19-39/23/24/25-23/24/25-11-36/38 | |||||||||
| Safety Statements | 24/25-59-61-9-46-16-45-36/37-7-26 | |||||||||
| RIDADR | UN 2831 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KJ2975000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29031910 | |||||||||
| Toxicity | Acute oral LD50 for dogs 750 mg/kg, guinea pigs 9,470 mg/kg, mice 11,240 mg/kg, rats 10,300 mg/kg, rabbits 5,660 mg/kg (quoted, RTECS, 1985). | |||||||||
| IDLA | 700 ppm | |||||||||
| NFPA 704 |
|
1,1,1-Trichloroethane Chemical Properties,Uses,Production
Description
1,1,1-Trichloroethane (1,1,1-TCE) was first identified in 1840 by Henri Victor Regnault, a French chemist and physicist. 1,1,1- TCE is a synthetic chemical that is released to the environment primarily by human industrial activity such as by-process and fugitive emissions during its manufacture, formulation, and use in both consumer and industrial products, which can then undergo thermal and photochemical chlorination. 1,1,1-TCE was originally introduced as a replacement for other chlorinated and flammable solvents like carbon tetrachloride. Although trichloroethane was formerly used extensively in a range of industrial applications and consumer products, including such products as adhesives and adhesive cleaners, lubricants, general purpose liquid cleaners and spray degreasers, oven cleaners, spot removers, shoe polish, and fabric finishes, and as a precursor for hydrofluorocarbons, it is no longer used in common household products. 1,1,1-TCE was one of the compounds addressed by the Montreal Protocol in 1987, which stipulates that the production and consumption of these potentially ozone-depleting substances in the stratosphere were to be phased out. Under this agreement, the final phase out for developed countries for 1,1,1-TCE was 1996, with selected exceptions for existing stocks and essential uses; developing countries have until 2015 for their ban to take effect.
Chemical Properties
1,1,1-Trichloroethane is a colorless liquid. It has an odor similar to chloroform. The Odor Threshold is 120 ppm (NJ) or 400 ppm (NY).
Chemical Properties
colourless liquid with a mild ether-like odour. Insoluble in water; soluble in alcohol and ether. Nonflammable.
Physical properties
Colorless, watery liquid with a dusty, sooty or polish-type odor similar to chloroform. At 40 °C, the average odor threshold concentration and the lowest concentration at which an odor was detected were 20,000 and 2,200 μg/L, respectively. At 25 °C, the lowest concentration at which a taste was detected was 1,500 μg/L, respectively (Young et al., 1996). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 0.47 and 0.32 mg/L, respectively (Alexander et al., 1982).
Uses
METHYL CHLOROFORM is used in industrial applications as a solvent and a degreasing agent.
Uses
Solvent for cleaning precision instruments, metal degreasing, pesticide, textile processing.
Uses
1,1,1-Trichloroethane is used as a cleaningsolvent for cleaning metals and plastic molds.
Production Methods
Most commercial methyl chloroform, which is sold under several trade names, contains inhibitors to prevent reaction of the solvent with aluminum and alloys. This reaction produces hydrogen chloride and in confined vessels may produce high pressures.
Definition
ChEBI: A member of the class of chloroethanes carrying three chloro substituents at position 1.
General Description
A colorless liquid with a sweet, pleasant odor. May irritate skin, eyes and mucous membranes. In high concentrations the vapors may have a narcotic effect. Nonflammable, but may decompose and emit toxic chloride fumes if exposed to high temperatures. Used as a solvent.
Air & Water Reactions
Insoluble in water. Absorbs some water.
Reactivity Profile
1,1,1-Trichloroethane decomposes in the presence of chemically active metals. This includes aluminum, magnesium and their alloys. 1,1,1-Trichloroethane will react violently with dinitrogen tetraoxide, oxygen, liquid oxygen, sodium and sodium-potassium alloys. 1,1,1-Trichloroethane will also react violently with acetone, zinc and nitrates. 1,1,1-Trichloroethane can react with sodium hydroxide. 1,1,1-Trichloroethane is incompatible with strong oxidizers and strong bases. Mixtures with potassium or its alloys are shock-sensitive and may explode on light impact. 1,1,1-Trichloroethane can react with an aqueous suspension of calcium hydroxide, and with chlorine in sunlight. 1,1,1-Trichloroethane will attack some forms of plastics, rubber and coatings. Upon contact with hot metal or on exposure to ultraviolet radiation, 1,1,1-Trichloroethane will decompose to form irritant gases. A cobalt/molybdenum-alumina catalyst will generate a substantial exotherm on contact with its vapor at ambient temperatures. Hazardous reactions also occur with (aluminum oxide + heavy metals). .
Health Hazard
INHALATION: symptoms range from loss of equilibrium and incoordination to loss of consciousness; high concentration can be fatal due to simple asphyxiation combined with loss of consciousness. INGESTION: produces effects similar to inhalation and may cause some feeling of nausea. EYES: slightly irritating and lachrymatory. SKIN: defatting action may cause dermatitis.
Health Hazard
The oral and inhalation toxicity of 1,1,1-trichloroethane is of low order in animalsand humans. It is an anesthetic at highconcentrations. Exposure to its vapors at a1.5% concentration in air may be lethal tohumans. Death may result from anesthesiaand/or cardiac sensitization. Prolonged skincontact may cause defatting and reddeningof eyes. Vapors are irritant to the eyes andmucous membranes.
The acute oral toxicity is low in testanimals. The oral LD50 values in rabbitsand guinea pigs are 5660 and 9470 mg/kg,respectively (NIOSH 1986). The carcino genicity of this compound in animals andhumans is not known.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating gases are generated in fires.
Industrial uses
Methyl chloroform is a versitile, all purpose solvent, popular with industry because of its powerful cleaning properties, low flammability, and low relative toxicity. It was introduced in the mid 1950s as a cold cleaning solvent substitute for carbon tetrachloride. Today, methyl chloroform is used primarily for vapor degreasing and cold cleaning of fabricated metal parts and other materials. The chemical also is used in fluoropolymer synthesis, as a solvent in adhesive and aerosol formulations, for the production of certain coatings and inks, for a variety of textile applications, and for dry cleaning leather and suede garments. Methyl chloroform is a member of a family of saturated aliphatic halogenated hydrocarbons.
Metal vapor degreasing is an important process in industrial manufacture, used to remove oils and oil-borne soils (i.e., chips, metal fines, and fluxes) from objects that have been stamped, machined, welded, soldered, molded, or diecast. Vapor degreased parts vary from tiny transistors to aircraft and spacecraft assemblies.
Methyl chloroform is an excellent solvent for the cold (room temperature) cleaning of a wide variety of manufacturing equipment and products including yarns, threads, finished cloth, reinforced fiberglass, plastics, and common and exotic metals. The solvent removes most greases, oils, lubricants, waxes, adhesives, inks, fluxes, paints, stamping and drawing compounds, tars, and other soils.
The main reasons for the use of methyl chloroform in formulations for urethane and neoprene/phenolic contact adhesives, mastics, sealants, and natural rubber tire repair cements are its ability to substantially reduce flammability, its nonphotochemical reactivity, and the favorable characteristics of the resulting adhesive formulation.
The main applications of methyl chloroform in the electronics industry are in circuit board fabrication, where it is used to develop dry film photoresist, and in the semiconductor industry where it is used for secondary cleaning.
Methyl chloroform serves as a raw material for the manufacture of polyvinylidene
fluoride fluoropolymer. It also can be used as a raw material for the production of
certain hydrochlorofluorocarbons having relatively short atmospheric residence
times.
In the coatings manufacturing industry, methyl chloroform is used as a solvent in
the formulation of protective and decorative coatings and as a thinner to reduce the
viscosity of high-solid content coatings for spray application. The chemical also can
be used in the production of rotogravure and flexographic inks. In addition to the
above uses, methyl chloroform is used to dry clean leather and suede products and
to clean motion picture film.
Contact allergens
Trichloroethane is a solvent that has wide applications in industry, such as for cold type metal cleaning and in cleaning plastic molds. It is mainly an irritant, but can also provoke allergic contact dermatitis.
Safety Profile
Poison by intravenous
route. Moderately toxic by ingestion,
inhalation, skin contact, subcutaneous, and
intraperitoneal routes. An experimental
teratogen. Human systemic effects by
ingestion and inhalation: conjunctiva
irritation, hallucinations or distorted
perceptions, motor activity changes,
irritability, aggression, hypermotility,
diarrhea, nausea or vomiting and other
gastrointestinal changes. Experimental
reproductive effects. Questionable
carcinogen. Mutation data reported. A
human skin irritant. An experimental skin
and severe eye irritant. Narcotic in high
concentrations. Causes a proarrhythmic
activity that sensitizes the heart to
epinephrine-induced arrhythmias. This
sometimes will cause cardlac arrest,
particularly when this material is massively
inhaled as in drug abuse for euphoria.
Under the proper conditions it can
undergo hazardous reactions with aluminum
oxide + heavy metals, dinitrogen tetraoxide,
inhbitors, metals (e.g., magnesium, aluminum, potassium, potassium-sodium
alloy), sodium hydroxide, N2O4, oxygen.
When heated to decomposition it emits
toxic fumes of Cl-. Used as a cleaning
solvent, as a chemical intermediate to
produce vinylidene chloride, and as a
propellant in aerosol cans.
Potential Exposure
1,1,1-Trichloroethane is used as a cleaning solvent, chemical intermediate for vinylidene chloride. In liquid form it is used as a degreaser and for cold cleaning, dip-cleaning; and bucket cleaning of metals. Other industrial applications of 1,1,1-trichlroethane’s solvent properties include its use as a dry-cleaning agent; a vapor degreasing agent; and a propellant. In recent years, 1,1,1-trichloroethane has found wide use as a substitute for carbon tetrachloride.
Carcinogenicity
IRIS provides a cancer
descriptor of “inadequate information to assess carcinogenic
potential.” This is based on inconclusive epidemiologic
studies. A 2 year inhalation bioassay showed no treatment-
related increase in tumors in rats and mice. The two
available oral cancer bioassays in rats and mice are inadequate
for evaluation of cancer potential. The compound has
been shown to be rather negative in short-term tests for
genotoxicity.
NCI tested rats and mice by oral and inhalation
routes, but the results were questionable.
Quast et al. exposed 96 Sprague–Dawley rats of both
sexes to 875 or 1750 ppm 1,1,1-trichloroethane vapor for 6 h/
day, 5 days/week for 12 months, followed by an additional
19 month observation period. The only significant sign of
toxicity was an increased incidence of focal hepatocellular
alterations in female rats at the highest dosage. Neither was it
evident that a maximum tolerated dose was used nor was a
range-finding study conducted. No significant dose-related
neoplasms were reported, but these dose levels were below
those used in the NCI study.
In another study, Quast et al. used an inhibited
formulation of 1,1,1-trichloroethane. Fischer 344 rats and
B6C3F1 mice of both sexes were exposed to 0, 150, 500, or
1500 ppm 6 h/day, 5 days/week for 2 years. The authors
indicate that there were no indications of an oncogenic effect on rats or mice following 2 years of exposure to the 1,1,1-
trichloroethane formulation and a NOAEL of 500 ppm for
adverse effect of any kind. The ATSDR reviewed this information
(52) and determined that the study adequately demonstrated
negative evidence of carcinogenicity in animals by
lifetime inhalation up to 1500 ppm.
Environmental Fate
Biological. Microbial degradation by sequential dehalogenation under laboratory conditions
produced 1,1-dichloroethane, cis- and trans-1,2-dichloroethylene, chloroethane, and vinyl
chloride. Hydrolysis products via dehydrohalogenation included acetic acid, 1,1-dichloroethylene
(Dilling et al., 1975; Smith and Dragun, 1984), and HCl (Dilling et al., 1975). The reported halflives
for this reaction at 20 and 25 °C are 0.5 to 2.5 and 1.1 yr, respectively (Vogel et al., 1987; ten
Hulscher et al., 1992).
Groundwater. Under aerobic conditions, 1,1,1-trichloroethane slowly degraded to 1,1-
dichloroethane (Parsons and Lage, 1985; Parson et al., 1985). Based on a study conducted by
Bouwer and McCarty (1984), the estimated half-life of 1,1,1-trichloroethane in groundwater three
months after injection was 200–300 d.
Surface Water. Estimated half-lives of 1,1,1-trichloroethane (4.3 μg/L) from an experimental
marine mesocosm during the spring (8–16 °C), summer (20–22 °C), and winter (3–7 °C) were 24,
12, and 11 d, respectively (Wakeham et al., 1983).
Photolytic. Reported photooxidation products include phosgene, chlorine, HCl, and carbon
dioxide (McNally and Grob, 1984). Acetyl chloride (Christiansen et al., 1972) and
trichloroacetaldehyde (U.S. EPA, 1975) have also been reported as photooxidation products.
1,1,1-Trichloroethane may react with OH radicals in the atmosphere producing chlorine atoms and
chlorine oxides (McConnell and Schiff, 1978). The rate constant for this reaction at 300 K is 9.0 x
10-9 cm3/molecule?sec (Hendry and Kenley, 1979).
Chemical/Physical. The evaporation half-life of 1,1,1-trichloroethane (1 mg/L) from water at 25
°C using a shallow-pitch propeller stirrer at 200 rpm at an average depth of 6.5 cm was 18.7 min
(Dilling, 1977).
Shipping
UN2831 1,1,1-Trichloroethane, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Wash it successively with conc HCl (or conc H2SO4), aqueous 10% K2CO3 (Na2CO3), aqueous 10% NaCl, dry it with CaCl2 or Na2SO4, and fractionally distil it. It can contain up to 3% dioxane as preservative. This is removed by washing successively with 10% aqueous HCl, 10% aqueous NaHCO3 and 10% aqueous NaCl, and distilling over CaCl2 before use. [Beilstein 1 IV 138.]
Toxicity evaluation
The central nervous system (CNS) is the most sensitive target
for 1,1,1-TCE, following inhalation exposure in animals and
humans. The CNS-depressant effects are thought to involve interactions of the parent compound with lipids and/or
proteins in neural membranes. In general, the lipophilic nature
of 1,1,1-TCE allows it to cross the blood–brain barrier readily
and partition into lipids in neuronal membranes, which ultimately
interfere with neural membrane function, bringing
about CNS depression, behavioral changes, and anesthesia.
High 1,1,1-TCE levels also produce depression of
respiration and blood pressure and cardiac arrhythmias by
sensitizing the heart to endogenous epinephrine. 1,1,1-TCE is
a weak hepatotoxicant, producing mild effects on the liver at
relatively high levels. The arrhythmogenic effects are thought to
be produced by the parent compound. It is uncertain whether
the hepatotoxic effects of 1,1,1-TCE are due to the parent
compound or are mediated by metabolites such as trichloroacetic
acid and/or reactive intermediates. In addition to the
depressive effect on the heart, membrane alterations are also
thought to play an important role in the occurrence of
arrhythmogenic effects. Incorporation of 1,1,1-TCE into the
membrane of cardiac myocytes near the gap junction is thought
to explain the observed inhibition of gap junction intercellular
communication in cardiac myocytes. Most of the effects of
1,1,1-TCE are hypothesized to be produced by the parent
compound, by interfering with the function of mitochondrial
and cellular membranes.
Incompatibilities
Not flammable under normal conditions. However, in close or closed spaces, it may form a dangerously explosive atmosphere. See also fireextinguishing section. Strong caustics; strong oxidizers; chemically active metals, such as aluminum, magnesium powder; sodium, potassium. Reacts slowly with water forming hydrochloric acid. Upon contact with hot metal or exposure to UV radiation, it will decompose to form hydrochloric acid, phosgene and dichloroacetylene. Forms shocksensitive mixtures with potassium or its alloys. Attacks natural rubber.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. As an alternative to disposal, trichloroethane may be recovered from waste gases and liquids from various processes and recycled.