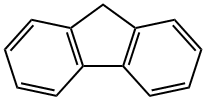
Fluorene
- CAS No.
- 86-73-7
- Chemical Name:
- Fluorene
- Synonyms
- 9H-FLUORENE;FLUOCINONIDE;Fluorene 98%;DIPHENYLENEMETHANE;uorene;FLUORENE;NSC 6787;Fluorene>Fluorene,98%;2,3-BENZINDENE
- CBNumber:
- CB3717011
- Molecular Formula:
- C13H10
- Molecular Weight:
- 166.22
- MDL Number:
- MFCD00001111
- MOL File:
- 86-73-7.mol
- MSDS File:
- SDS
| Melting point | 111-114 °C (lit.) |
|---|---|
| Boiling point | 298 °C (lit.) |
| Density | 1.2 |
| vapor pressure | 13 hPa (146 °C) |
| refractive index | 1.6470 |
| Flash point | 151 °C |
| storage temp. | room temp |
| solubility | 0.002g/l insoluble |
| form | Crystalline Powder |
| pka | >15 (Christensen et al., 1975) |
| color | Almost white to light brown |
| Water Solubility | insoluble |
| Merck | 14,4155 |
| BRN | 1363491 |
| Henry's Law Constant | 1.89(x 10-5 atm?m3/mol) at 4 °C, 12.5 at 25 °C (dynamic equilibrium method, Bamford et al., 1999) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | NIHNNTQXNPWCJQ-UHFFFAOYSA-N |
| LogP | 4.164 |
| CAS DataBase Reference | 86-73-7(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 3Q2UY0968A |
| IARC | 3 (Vol. Sup 7, 92) 2010 |
| NIST Chemistry Reference | Fluorene(86-73-7) |
| EPA Substance Registry System | Fluorene (86-73-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H410 | |||||||||
| Precautionary statements | P273-P391-P501 | |||||||||
| Hazard Codes | N,T,F,Xn,Xi | |||||||||
| Risk Statements | 50/53-39/23/24/25-23/24/25-11-67-65-38-36/38-36/37/38-52/53-20 | |||||||||
| Safety Statements | 60-61-24/25-45-36/37-16-7-62-33-24-22-36/37/39-27-26-25-9 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LL5670000 | |||||||||
| Hazard Note | Harmful | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29029080 | |||||||||
| Toxicity | Drinking water standard: No MCLGs or MCLs have been proposed, however, a DWEL of 1 mg/L was recommended (U.S. EPA, 2000). | |||||||||
| NFPA 704 |
|
Fluorene price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 128333 | Fluorene 98% | 86-73-7 | 5g | $41.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 48568 | Fluorene analytical standard | 86-73-7 | 5000mg | $74.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 128333 | Fluorene 98% | 86-73-7 | 100g | $92.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.20572 | Fluorene for synthesis | 86-73-7 | 100g | $97 | 2022-05-15 | Buy |
| Sigma-Aldrich | 8.20572 | Fluorene for synthesis | 86-73-7 | 820572 | $1877 | 2022-05-15 | Buy |
Fluorene Chemical Properties,Uses,Production
Description
Fluorene, when pure, is found as dazzlingwhite flakes or small, crystalline plates. It is fluorescentwhen impure. Polynuclear aromatic hydrocarbons (PAHs)are compounds containing multiple benzene rings and arealso called polycyclic aromatic hydrocarbons. Molecularweight=166.23; Boiling point=293C (decomposes);Freezing/Melting point=116-117C; Flash point=151C. Hazard Identification (based on NFPA-704 MRating System): Health 1, Flammability 1, Reactivity 0.Insoluble in water.
Chemical Properties
white crystals
Chemical Properties
Fluorene, when pure, is found as dazzling white flakes or small, crystalline plates. It is fluorescent when impure. Polycyclic aromatic hydrocarbons (PAHs) are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons.
Physical properties
Small white leaflets or crystalline flakes from ethanol. Fluorescent when impure.
Uses
Fluorene was used study the extraction of specific, semiconducting single-wall carbon nanotubes (SWCNTs).
Uses
Polycyclic aromatic hydrocarbons as micropollutants.
Definition
ChEBI: An ortho-fused tricyclic hydrocarbon that is a major component of fossil fuels and their derivatives
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 2656, 1951 DOI: 10.1021/ja01150a069
Synthetic Communications, 26, p. 1467, 1996 DOI: 10.1080/00397919608003512
The Journal of Organic Chemistry, 37, p. 1273, 1972 DOI: 10.1021/jo00973a049
General Description
White leaflets. Sublimes easily under a vacuum. Fluorescent when impure.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as Fluorene, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.
Hazard
Questionable carcinogen.
Health Hazard
ACUTE/CHRONIC HAZARDS: Fire hazards: Slight, when exposed to heat or flame.
Health Hazard
Acute toxicity in animals is very low. AnLD50 (intraperitoneal) in mice is 2000 mg/kg.Carcinogenicity of this compound in animalsis not well established. It tested negative in ahistidine reversion–Ames test.
Potential Exposure
Fluorene is used in resins, dyes, and is a chemical intermediate.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Source
Fluorene was detected in groundwater beneath a former coal gasification plant in Seattle,
WA at a concentration of 140 μg/L (ASTR, 1995). Present in diesel fuel and corresponding aqueous phase (distilled water) at concentrations of 350 to 900 mg/L and 12 to 26 g/L,
respectively (Lee et al., 1992). Schauer et al. (1999) reported fluorene in diesel fuel at a
concentration of 52 g/g and in a diesel-powered medium-duty truck exhaust at an emission rate of
34.6 g/km. Diesel fuel obtained from a service station in Schlieren, Switzerland contained fluorene
at an estimated concentration of 170 mg/L (Schluep et al., 2001).
Based on laboratory analysis of 7 coal tar samples, fluorene concentrations ranged from 1,100 to
12,000 ppm (EPRI, 1990). Lao et al. (1975) reported a fluorene concentration of 27.39 g/kg in a
coal tar sample. Detected in 1-yr aged coal tar film and bulk coal tar at an identical concentration
of 4,400 mg/kg (Nelson et al., 1996). A high-temperature coal tar contained fluorene at an average
concentration of 0.64 wt % (McNeil, 1983). Identified in high-temperature coal tar pitches at
concentrations ranging from 800 to 4,000 mg/kg (Arrendale and Rogers, 1981). Lee et al. (1992a)
equilibrated 8 coal tars with distilled water at 25 °C. The maximum concentration of fluorene
observed in the aqueous phase was 0.3 mg/L.
Fluorene was detected in asphalt fumes at an average concentration of 34.95 ng/m3 (Wang et al.,
2001).
Nine commercially available creosote samples contained fluorene at concentrations ranging
from 19,000 to 73,000 mg/kg (Kohler et al., 2000).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
625. Average fluorene concentrations reported in water-soluble fractions of unleaded gasoline,
kerosene, and diesel fuel were 1, 3, and 10 μg/L, respectively.
Fluorene was detected in soot generated from underventilated combustion of natural gas doped
with toluene (3 mole %) (Tolocka and Miller, 1995).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rates of fluorene were 4.44 mg/kg of pine burned, 3.83 mg/kg of oak burned, and 2.613 mg/kg of
eucalyptus burned.
California Phase II reformulated gasoline contained fluorene at a concentration of 4.35 mg/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 9.72 and 358 μg/km, respectively (Schauer et al., 2002).
Under atmospheric conditions, a low rank coal (0.5–1 mm particle size) from Spain was burned
in a fluidized bed reactor at seven different temperatures (50 °C increments), beginning at 650 °C.
The combustion experiment was also conducted at different amounts of excess oxygen (5 to 40%)
and different flow rates (700 to 1,100 L/h). At 20% excess oxygen and a flow rate of 860 L/h, the
amount of fluorine emitted ranged from 850.7 ng/kg at 950 °C to 3,632.8 ng/kg at 750 °C. The
greatest amount of PAHs emitted were observed at 750 °C (Mastral et al., 1999).
In one study, fluorene comprised about 7.6% of polyaromatic hydrocarbons in creosote (Grifoll
et al., 1995).
Identified as an impurity in commcerially available acenaphthene (Marciniak, 2002).
Typical concentration of fluorene in a heavy pyrolysis oil is 1.6 wt % (Chevron Phillips, May
2003).
Environmental Fate
Biological. Fluorene was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum. Significant biodegradation with gradual adaptation was observed.
At concentrations of 5 and 10 mg/L, biodegradation yields at the end of 4 wk of incubation were
77 and 45%, respectively (Tabak et al., 1981).
Photolytic. Fluorene reacts with photochemically produced OH radicals in the atmosphere. The
atmospheric half-life was estimated to range from 6.81 to 68.1 h (Atkinson, 1987). Behymer and
Hites (1985) determined the effect of different substrates on the rate of photooxidation of fluorene
(25 μg/g substrate) using a rotary photoreactor. The photolytic half-lives of fluorene using silica
gel, alumina, and fly ash were 110, 62, and 37 h, respectively. Gas-phase reaction rate constants
for OH radicals, NO3 radicals, and ozone at 24 °C were 1.6 x 10-11, 3.5 x 10-15, and <2 x 10-19 in
cm3/molecule?sec, respectively (Kwok et al., 1997).
Chemical/Physical. Oxidation by ozone to fluorenone has been reported (Nikolaou, 1984).
Chlorination of fluorene in polluted humus poor lake water gave a chlorinated derivative
tentatively identified as 2-chlorofluorene (Johnsen et al., 1989). This compound was also
identified as a chlorination product of fluorene at low pH (<4) (Oyler et al., 1983). It was
suggested that the chlorination of fluorene in tap water accounted for the presence of
chlorofluorene (Shiraishi et al., 1985).
storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from oxidizers and source of ignition.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz ardous material, Technical Name Required.
Purification Methods
Purify fluorene by chromatography of CCl4 or pet ether (b 40-60o) solution on alumina, with *benzene as eluent. Crystallise it from 95% EtOH, 90% acetic acid and again from EtOH. Crystallisation using glacial acetic acid retains an impurity which is removed by partial mercuration and precipitation with LiBr [Brown et al. J Am Chem Soc 84 1229 1962]. It has also been crystallised from hexane, or *benzene/EtOH, distilled under vacuum and purified by zone refining. [Gorman et al. J Am Chem Soc 107 4404 1985, Beilstein 5 IV 2142.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Compound can react exo thermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel Crafts reaction.
Waste Disposal
Persons in charge of vessels or facilities are required to notify the National Response Center (NRC) immediately when there is a release of this designated hazardous substance, in an amount equal to or greater than its RQ listed above. The toll free number of the NRC is (800) 424-8802; In the Washington D.C. metro politan area call (202) 426-2675. The rule for determining when notification is required is stated in 40 CFR 302.4 (Section IV. D.3.b).
Fluorene Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Mascot I.E.Co .,Ltd. | China | 303 | 58 | ||
| Jiangsu Guotai Guomian Trading Co. Ltd. | +86-512-58916079 +86-13601562190 | pharmachem@gtgmt.com | China | 148 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
View Lastest Price from Fluorene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-11-04 | Fluorene
86-73-7
|
US $20.00-1.00 / kg | 1kg | 0.99 | 10 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-10-09 | Fluorene
86-73-7
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-09-06 | Fluorene
86-73-7
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
86-73-7(Fluorene)Related Search:
1of4