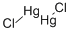MERCUROUS CHLORIDE
- CAS No.
- 10112-91-1
- Chemical Name:
- MERCUROUS CHLORIDE
- Synonyms
- MERCURY(II) CHLORIDE;MERCURY(I) CHLORIDE;Hg2-Cl2;Calotab;CALOMEL;Calogreen;mercurous;chloromercury;chloruremercureux;Chlorure mercureux
- CBNumber:
- CB3718285
- Molecular Formula:
- Cl2Hg2
Lewis structure

- Molecular Weight:
- 472.09
- MDL Number:
- MFCD00011043
- MOL File:
- 10112-91-1.mol
| Melting point | 400 °C (subl.)(lit.) |
|---|---|
| Boiling point | 383°C |
| Density | 7.15 |
| vapor pressure | 1.7 mm Hg at 236 °C |
| storage temp. | Poison room |
| solubility | insoluble in ethanol, ethyl ether |
| form | powder |
| Specific Gravity | 7.15 |
| color | White |
| Odor | Odorless |
| Water Solubility | Soluble in (0.002g/L )water. |
| Sensitive | Moisture & Light Sensitive |
| Merck | 14,5894 |
| Solubility Product Constant (Ksp) | pKsp: 17.88(25°C) |
| Exposure limits |
ACGIH: TWA 0.025 mg/m3 (Skin) NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| Dielectric constant | 7.0(Ambient) |
| InChIKey | ZOMNIUBKTOKEHS-UHFFFAOYSA-L |
| CAS DataBase Reference | 10112-91-1(CAS DataBase Reference) |
| FDA 21 CFR | 310.545 |
| EWG's Food Scores | 1 |
| FDA UNII | J2D46N657D |
| NIST Chemistry Reference | Mercurous chloride(10112-91-1) |
| EPA Substance Registry System | Mercurous chloride (10112-91-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312-H315-H319-H334-H335-H410-H302-H400 | |||||||||
| Precautionary statements | P280a-P405-P501a-P261-P280-P284-P304+P340-P305+P351+P338-P342+P311 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 22-36/37/38-50/53 | |||||||||
| Safety Statements | 13-24/25-46-60-61 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OV8740000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
MERCUROUS CHLORIDE price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Alfa Aesar | 087240 | Mercury(I) chloride, 99.5% | 10112-91-1 | 100g | $71.9 | 2023-06-20 | Buy |
| Alfa Aesar | 036419 | Mercury(I) chloride, ACS, 99.5% min | 10112-91-1 | 100g | $84.4 | 2023-06-20 | Buy |
| Strem Chemicals | 93-8018 | Mercury(I) chloride, 99.5+% (ACS) | 10112-91-1 | 100g | $154 | 2024-03-01 | Buy |
| Strem Chemicals | 93-8018 | Mercury(I) chloride, 99.5+% (ACS) | 10112-91-1 | 500g | $613 | 2024-03-01 | Buy |
| Alfa Aesar | 087240 | Mercury(I) chloride, 99.5% | 10112-91-1 | 500g | $271 | 2023-06-20 | Buy |
MERCUROUS CHLORIDE Chemical Properties,Uses,Production
Chemical Properties
Mercury (I) chloride is a dense white powder and insoluble in water and may be light sensitive. It is incompatible with strong bases, carbonates, sulphides, cyanides, alkalis, sulphites, sulphates, hydrogen peroxide, ammonia, iodine, and hydrogen bromide.
Uses
Mercury(I) chloride is used in calomel electrodes; in ceramic painting; as a fungicide; in pyrotechnics for producing dark green light; in agriculture for controlling root maggots; and as an antiseptic and antisyphilitic agent in medicine.
Preparation
Mercury(I) chloride is prepared by passing a limited amount of chlorine gas over mercury in a heated silica retort. Excess chlorine should be avoided as it can oxidize mercury(I) chloride to mercury(II) chloride.
2Hg + Cl2 → Hg2Cl2
The product generally contains some mercury(II) chloride which is removed by treating the product mixture with water and filtering out the insoluble mercury(I) salt from the soluble mercury(II) salt.
The compound also can be made by heating mercury(II) chloride with mercury. The product Hg2Cl2 sublimes and is collected:
HgCl2 + Hg → Hg2Cl2
Mercury(I) chloride is obtained as a white precipitate by adding a cold acidic solution of sodium chloride or other soluble chloride to a solution of mercurous salt, such as mercury(I) nitrate:
[Hg2]2+ (aq) + 2Cl¯ (aq) → Hg2Cl2 (s)
The precipitation method, however, does not form high-purity product as it contains small amounts of reactant and product ions that stick to the Hg2Cl2 precipitate and are difficult to remove by washing with water.
Reactions
Mercury(I) chloride oxidizes to mercury(II) chloride when heated with chlorine:
Hg2Cl2 + Cl2 → 2HgCl2
Mercury(I) chloride is a reducing agent and, therefore, its reaction with oxidizing substances can oxidize it to Hg(II) compounds.
When heated at elevated temperatures, it partially dissociates to mercury metal and mercury(II) chloride:
Hg2Cl2 → Hg + HgCl2
This disproportionation (or breakdown of a compound into two products containing the same element but in different oxidation states) also occurs to some degree when mercury(I) chloride is heated and sublimed in an open container. Reaction with ammonia in solution forms an unstable black adduct which slowly converts to mercury(II) amidochloride, NH2HgCl, releasing mercury:
Hg2Cl2 + 2NH3 → ClHg—HgNH2 + NH4Cl
ClHg—HgNH2 → NH2HgCl + Hg
Toxicity
Mercury(I) chloride is highly toxic by ingestion and other routes of exposure. The symptoms include nausea, vomiting, abdominal pain, diarrhea and kidney damage.
Chemical Properties
White, rhombic crystals or crystalline powder; odorless. Stable in air but darkens on exposure to light.decomposed by alkalies. Insoluble in water, ether, alcohol, and cold dilute acids.
Chemical Properties
Mercurous chloride forms tetrahedral white crystals, and, unlike the mercuric salt, is only very slightly soluble in water (about 2mg/L water at 20°C).
Uses
Calomel is used as a laboratory reagent, as a fungicide, and as a depolarizer in dry batteries.
Uses
This compound is used by the pharmaceutical industry and is used also as a fungicide, as a poison, in fireworks, and to control maggots.
Uses
Dark green Bengal lights; calomel paper, mixed with gold in painting on porcelain; for calomel electrodes; as fungicide; in agriculture to control root maggots on cabbage and onions.
Production Methods
Mercurous chloride is produced by exposing mercury metal to limited amounts of chlorine gas, insufficient to form mercuric chloride as the major product; it can also be prepared by precipitation from mercurous nitrate solution.
Definition
ChEBI: Dimercury dichloride is a mercury coordination entity.
General Description
Odorless white solid. Sinks in water.
Reactivity Profile
MERCUROUS CHLORIDE is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper .
Hazard
Toxic dose is uncertain.
Health Hazard
Acute poisoning can result from inhaling dust concentrations of 1.2-8.5 mg/m 3 in air; symptoms include pain and tightness in chest, coughing, and difficulty in breathing. Compound is an irritant, cathartic, or purgat ive; rarely, ``calomel sickness,'' a benign reaction with fever and rash, appears after about 1 week; seldom causes systemic poisoning but may be fatal if retained to 30-40 mg/kg. Contact with eyes causes mild irritation.
Carcinogenicity
An acute oral dose in humans of 1 g HgCl2 may cause corrosive damage to the GI tract; there is, however, little quantitative information on dose–effect relationships during low-level exposure to inorganic mercury. A dose of 2 g may be expected to increase mortality greatly among victims of the poison. Death from acute oral exposure is usually caused by cardiovascular collapse and renal failure. Ingestion of inorganic compounds may cause gastrointestinal corrosion and irritation, such as vomiting, bloody diarrhea, and stomach pains.
Environmental Fate
Calomel can generate reactive oxygen species and deplete glutathione levels. Both genotoxic and nongenotoxic mechanisms may contribute to renal carcinogenic effect of mercury.
Toxicity evaluation
Calomel decomposes gradually in the presence of sunlight. It slowly decomposes to mercury and mercuric chloride under aqueous conditions.
MERCUROUS CHLORIDE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | sales@tjzxchem.com | China | 559 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29322 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9165 | 58 |
| GLR Innovations | +91 9891111994 | info@glrgroup.in | India | 4542 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
Related articles
- Side effects of Calomel
- Calomel is a mercury chloride mineral with formula Hg2Cl2 (see mercury(I) chloride). The name derives from Greek kalos (beauti....
- Dec 6,2021
View Lastest Price from MERCUROUS CHLORIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-22 | MERCUROUS CHLORIDE USP/EP/BP
10112-91-1
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2020-05-04 | Mercury(I) chloride
10112-91-1
|
US $0.00-0.00 / KG | 1KG | 99.0% | 600 Tons | Shaanxi Dideu Medichem Co. Ltd |
-

- MERCUROUS CHLORIDE USP/EP/BP
10112-91-1
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- Mercury(I) chloride
10112-91-1
- US $0.00-0.00 / KG
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
10112-91-1(MERCUROUS CHLORIDE)Related Search:
1of4