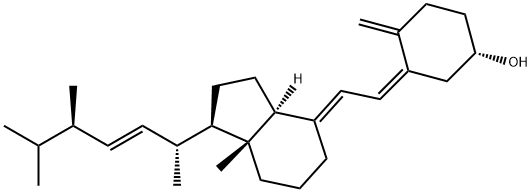
Vitamin D2
- CAS No.
- 50-14-6
- Chemical Name:
- Vitamin D2
- Synonyms
- ERGOCALCIFEROL;VITAMIN D;CALCIFEROL;Calderol;VitaMin D2-d3;Drisdol;Doral;Crtron;Deltalin;Sterogyl
- CBNumber:
- CB3731934
- Molecular Formula:
- C28H44O
- Molecular Weight:
- 396.65
- MDL Number:
- MFCD00166988
- MOL File:
- 50-14-6.mol
- MSDS File:
- SDS
| Melting point | 114-118 °C(lit.) |
|---|---|
| alpha | 82 º (c=3, in acetone 25 ºC) |
| Boiling point | 460.3°C (rough estimate) |
| Density | 0.9784 (rough estimate) |
| vapor pressure | 7 x l0-7 Pa (20 °C), est.) |
| refractive index | 1.5100 (estimate) |
| Flash point | 14 °C |
| storage temp. | -20°C |
| solubility | H2O: 200 mg/mL, clear to hazy |
| form | powder |
| pka | 14.74±0.20(Predicted) |
| color | white to yellowish |
| optical activity | [α]20/D +105±2°, c = 4% in ethanol |
| Water Solubility | Soluble in ethanol, water, methanol, dimethylformamide, and dimethyl sulfoxide. |
| Merck | 14,10018 |
| BRN | 1916682 |
| BCS Class | 3 |
| Stability | Light Sensitive |
| InChIKey | MECHNRXZTMCUDQ-RKHKHRCZSA-N |
| LogP | 10.440 (est) |
| FDA 21 CFR | 172.379; 310.545 |
| Substances Added to Food (formerly EAFUS) | VITAMIN D-2 |
| SCOGS (Select Committee on GRAS Substances) | Vitamin D2 (ergocalciferol) |
| CAS DataBase Reference | 50-14-6(CAS DataBase Reference) |
| EWG's Food Scores | 2-5 |
| NCI Dictionary of Cancer Terms | ergocalciferol; vitamin D2 |
| FDA UNII | VS041H42XC |
| NCI Drug Dictionary | ergocalciferol |
| ATC code | A11CC01 |
| NIST Chemistry Reference | Ergocalciferol(50-14-6) |
| EPA Substance Registry System | Ergocalciferol (50-14-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311-H330-H372 | |||||||||
| Precautionary statements | P260-P264-P280-P302+P352+P312-P304+P340+P310-P314 | |||||||||
| Hazard Codes | Xn,T+,T,F | |||||||||
| Risk Statements | 22-48/25-26-24/25-40-23/24/25-48/22-23-11-20 | |||||||||
| Safety Statements | 28-36/37-45-28A-36-26-36/37/39-22-16-7 | |||||||||
| RIDADR | UN 2811 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KE1050000 | |||||||||
| F | 1-8-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29362900 | |||||||||
| NFPA 704 |
|
Vitamin D2 price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | V-024 | Vitamin D2 solution 1?mg/mL in ethanol, ampule of 1?mL, certified reference material, Cerilliant? | 50-14-6 | 1mL | $103 | 2024-03-01 | Buy |
| Sigma-Aldrich | 95220 | Ergocalciferol ≥98.0% (sum of enantiomers, HPLC) | 50-14-6 | 1g | $95.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1239005 | Ergocalciferol United States Pharmacopeia (USP) Reference Standard | 50-14-6 | 5x30mg | $483 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP788 | Ergocalciferol British Pharmacopoeia (BP) Reference Standard | 50-14-6 | 500MG | $259 | 2023-06-20 | Buy |
| TCI Chemical | C0005 | Calciferol >98.0%(HPLC) | 50-14-6 | 1g | $63 | 2024-03-01 | Buy |
Vitamin D2 Chemical Properties,Uses,Production
Description
Ergocalciferol, also under the name vitamin D2, is a type of vitamin D found in plants and yeast. It has no antirachitic activity. It helps the body to absorb calcium and phosphorus. It is used as a dietary supplement. It is used in the treatment of hypoparathyroidism (a condition in which not enough parathyroid hormone can be produced by the body), refractory rickets (softening and weakening of bones that does not respond to treatment, it is also known as vitamin D resistant rickets), and familial hypophosphatemia (rickets or osteomalacia caused by an inherited condition with a decreased ability to break down vitamin D in the body). Ergocalciferol is also used as a rodenticide.
Vitamin D
Vitamin D refers to a group of fat-soluble steroid compounds with anti-rickets effect and is called anti-rickets vitamin. It is known currently about at least ten or more sterols substance with vitamin D activity. They are mainly presented in the vegetable oil or yeast ergosterol solid which subject to sunlight or ultraviolet irradiation to generate vitamin D2 which is also called as calciferol or ergocalciferol. Therefore, ergosterol is also called as original vitamin D2; the other is from that the human body can convert the cholesterol to the 7-dihydro-cholesterol to be stored in the skin with the later being able to be converted to vitamin D3 upon sunlight and ultraviolet radiation. Vitamin D3 is also known as cholecalciferol and therefore the 7-dehydrocholesterol is also known as original vitamin D3. Vitamin D2 has a very similar structure with vitamin D3 and both of them are the B ring open-ring derivatives of precursor sterols with the difference of them being laid in a extra double bond and a methyl group in the side chain of vitamin D2.
Both vitamin D2 and vitamin D3 are colorless or white crystals and are not foul with the melting point being 115~118 ℃ and 84~85 ℃, respectively. They are insoluble in water and easily soluble in alcohol, ether, acetone, and slightly soluble in vegetable oil. They are unstable in air and sunlight and are easily inactivated in moist air. However, vitamin D3 is relatively more stable than vitamin D2. These two have similar physiological roles in the human body with the major role being maintain the normal metabolism of calcium and phosphorus, promoting the deposition of calcium and phosphorus into the bone and tissue and thus it can be used in prevention and treatment of metabolic bone disease such as rickets and osteomalacia.
Vitamin D2 and D3 have the same effects on mammals and cows and pigs. However, vitamin D3 has a ten times stronger activity on poultry (birds) than vitamin D2.
Vitamin D deficiency can reduce the intestinal absorption of calcium and cause the decomposition of bone calcium and phosphorus. Young livestock will get osteomalacia and adult animals are prone to osteoporosis. Vitamin D deficiency can also lead to animal sternum and spine deformation and that layers give birth to soft-shell eggs. Because vitamin D control the absorption of calcium, phosphorus, too much absorption of vitamin D in the diet can cause high blood calcium, so that excess calcium can be deposited in the heart, blood vessels, joints, pericardium or intestinal wall, leading to heart failure, joint stiffness or intestines disorders.
Sunbathing is the most economical source of getting vitamin D. The ergosterol or 7-dehydrocholesterol can be converted into vitamin D inside animal body upon ultraviolet radiation. The hay, yeast, corn leaves, barley, oats, wheat under sunshine and yeast which has been subject to ultraviolet treatment is a good source of vitamin D. Animal products can directly provide vitamin D for the livestock, such as eggs, milk. Cod liver oil is rich in vitamin D levels. Reared animals and animal with insufficient sunlight need to have their daily feed supplied with additional vitamin D.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Chemical Properties
It is white needle crystal or crystalline powder and is odorless and tasteless. Its melting point is 115-118 ℃ (decomposition), Its specific rotation is [α]20D+102.5°(ethanol). The ethanol solution of this product has maximum absorption at 265nm wavelength. It is soluble in ethanol (1: 2), diethyl ether (1: 2), acetone (1:10) and chloroform (1: 0.7) but insoluble in water. Moreover, it has a lower activity in case of light or oxygen.
Uses
It can be applied to biochemical studies; its clinical drug belongs to a lipid-soluble vitamin which can promote the intestinal absorption of calcium and phosphorus which plays a role of facilitating the calcification of bone. It is clinically used for prevention and treatment of rickets in children and adult osteomalacia.
Vitamin D2 can maintain the normal metabolism of calcium and phosphorus and can also promote the body's absorption of calcium and phosphorus. Upon its deficiency, the children are easy to get rickets. China ruled that it can be applied for strengthening margarine with the usage amount being 125~156 μg/kg; its usage amount in fortified dairy is 63~125μg/kg; the usage amount in strengthening infant and children food used is 50~100μg/kg; the usage amount in strengthening milk or milk beverage is 10~40μg/kg; the maximal allowable usage amount in the strengthening solid drinks and ice cream is 10~20μg/kg.
It is mainly applied to the prevention and treatment of rickets, osteomalacia, and infant tetany psychosis.
Toxicity
Acute toxicity dose: 100 mg/d (adult, orally). Mouse lethal dose of 20mg/kg (6 Day). Excess can easily cause poisoning.
GRAS (FDA, §182.5950, 2000).
LD50: 42mg/kg (rat, oral).
Limited use
GB 14880-94 (μg/kg): liquid milk from 10 to 20; margarine: 125 to 156; dairy products: 63 to 125; milk and milk drinks: 10 to 40; infant and children food: 50 to 115.
GB 2760-2002 (pg/kg): solid drink, ice cream, 10 to 20; arrowroot flour: 50 to 100; Soy milk, soy flour, 15 to 60; soy milk, soy milk, 3 to 15; jelly: 10 to 40; instant breakfast cereals, 12.5 to 37.5; puffed food sandwich from 10 to 60.
FDA, §184.1950 (IU/100g; 2001): breakfast cereals 350; 90 granular and pasty products; milk 42; 89 dairy products.
Production method
Dissolve the ergosterol dissolved in ethanol and go through ring-opening under UV illumination; the reaction was further concentrated under reduced pressure, frozen, filtered, and filtered of liquid nitrogen, and concentrated under reduced pressure to dryness to obtain the crude oil of vitamin D2 with distillation to obtain the refined products.
Vitamin D2 is naturally presented in the liver, egg yolk and milk; the production method of the industry starts with extracting the ergocalciferol from vegetable oil or yeast extract in the body. Further dissolve it in chloroform or cyclohexane, and then convert it to the vitamin D2 through ultraviolet radiation on the quartz glass flask.
Ergosterol ethanol solution is subject to ultraviolet radiation with the 9’, 10’ bond breakage to obtain the vitamin D2 crude product; the later one further has esterification with 3, 5-nitrobenzoyl chloride and undergoes alkaline hydrolysis to get the purified product.
References
- https://en.wikipedia.org/wiki/Ergocalciferol
- https://pubchem.ncbi.nlm.nih.gov/compound/ergocalciferol
- https://www.drugs.com
- https://medlineplus.gov
- https://www.webmd.com
Description
Vitamin D aids in the absorption of calcium and has central roles in bone formation and maintenance, hypertension, cancer and immunity. Vitamin D may be obtained from many dietary sources, including eggs and fish, and is synthesized in the skin by the conversion of 7-
Chemical Properties
Ergocalciferol is an odorless white crystalline solid
Uses
The synthetic form of vitamin D. Prepared from ergosterol by UV irradiation in a suitable solvent. Commercial solutions are usually made with propylene glycol or sesame oil. Antirachitic
Uses
Vitamin d2 is fat-soluble, and is stable unless oxidized. It is necessary for growth and maintenance of teeth and bones and the normal utilization of calcium and phosphorus; it is used medicinally in the treatment of rickets and as a dietary supplement. Its sources include fish liver and vitamin d-fortified milk.
Uses
Rodenticide.
Uses
Vitamin D2 is formed by photochemical cleavage of ergosterin, which is a side-product of many fermentation processes. Microorganisms usually contain up to 3 percent of ergosterin.
Uses
antirachitic vitamin; LD50 (rat) 56 mg/kg po
Definition
ChEBI: A vitamin D supplement and has been isolated from alfalfa.
brand name
Deltalin (Lilly); Drisdol(Sanofi Aventis).
General Description
Odorless white crystals. Used as a dietary supplement and food additive.
Reactivity Profile
Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.
Health Hazard
Vitamin D2 poisoning disturbs calcium metabolism and causes kidney damage. Vitamin D2 in a single acute ingestion presents no toxic hazards. Daily ingestion in excess of 5000 units/day in children or 7500 units/day in adults will produce toxic symptoms associated with hypervitaminosis D. Those with hypercalcemia are at a greater risk.
Fire Hazard
Shows signs of decomposition when stored for a few days at room temperature.
Biochem/physiol Actions
Ergocalciferol (vitamin D2) and 25-Hydroxycholecalciferol (vitamin D3) are the two form of vitamin D which are activated in vivo by hydroxylation. Vitamin D2 and D3 may be used in a wide range of studies to assess their effects on function such as immune function and calcium homeostasis.
Safety Profile
Poison by ingestion, intraperitoneal, intravenous, and intramuscular routes. An experimental teratogen. Human systemic effects by ingestion: anorexia, nausea or vomiting, and weight loss. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Used as a nutrient and/or dietary supplement food additive
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Metabolic pathway
Ergocalciferol (vitamin D2) is a product made by the UV irradiation of yeast ergosterol. It is a common form of vitamin D and it has very similar biological activity to that of cholecalciferol (vitamin D3) in the treatment of the vitamin D deficiency diseases (e.g. rickets). The bioactive form of this vitamin is also the 1,25-dihydroxy-derivative and this has equal antirachitic activity to that of the cholecalciferol analogue (Jones et al., 1975).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, well-ventilated area.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
It is converted into its 3,5-dinitrobenzoyl ester and crystallised repeatedly from acetone. The ester is then saponified and the free vitamin is isolated. [Laughland & Phillips Anal Chem 28 817 1956, Beilstein 6 IV 4404.]
Degradation
It is unstable in light and air and in acidic media. It is inactivated within a few days under normal exposure conditions. This is due to oxidation and fragmentation of the triene functionality. Ergocalciferol is slightly more unstable, probably because of the additional alkene group in the side chain. One of the several degradation products of ergocalciferol has been identified as the ketone (8, shown in Scheme 1) formed by the loss of the methano-cyclohexanol function (Stewart et al., 1984).
Toxicity evaluation
Excess vitamin D in the form of 1,25-dihydroxycalciferol results in hypercalcemia and hypercalciuria, due to increased calcium absorption, bone demineralization, and hyperphosphatemia.
Incompatibilities
Dust may be combustible and may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides.
Waste Disposal
Dispose of contents and container to an approved waste disposal plant. All federal, state, and local environmental regulations must be observed. It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffeegrounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Vitamin D2 Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 874 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
View Lastest Price from Vitamin D2 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Vitamin D2
50-14-6
|
US $10.00 / kg | 1kg | Food Grade | 10000 | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-04-22 | Vitamin D2
50-14-6
|
US $85.00-35.00 / Kg/Bag | 1Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-11 | Vitamin D2
50-14-6
|
US $0.00 / kg | 1kg | 99% | 1000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd |
-

- Vitamin D2
50-14-6
- US $10.00 / kg
- Food Grade
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- Vitamin D2
50-14-6
- US $85.00-35.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- Vitamin D2
50-14-6
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
50-14-6(Vitamin D2)Related Search:
1of4