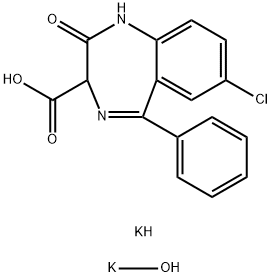
CLORAZEPATE DIPOTASSIUM SALT
- CAS No.
- 57109-90-7
- Chemical Name:
- CLORAZEPATE DIPOTASSIUM SALT
- Synonyms
- Azene;cm4306;cb4306;4306cb;ah3232;mendon;ab35616;latekoh;tr19119;belseren
- CBNumber:
- CB3737547
- Molecular Formula:
- C16H13ClK2N2O4
- Molecular Weight:
- 410.94
- MDL Number:
- MFCD00063405
- MOL File:
- 57109-90-7.mol
| Melting point | 228-235°C |
|---|---|
| Flash point | 11 °C |
| storage temp. | 2-8°C |
| solubility | Freely soluble to very soluble in water, very slightly soluble in alcohol, practically insoluble in methylene chloride. Solutions in water and in alcohol are unstable and are to be used immediately. |
| form | Solid |
| color | White to Pale Yellow |
| FDA UNII | 63FN7G03XY |
| Proposition 65 List | Clorazepate Dipotassium |
| EPA Substance Registry System | Clorazepate dipotassium (57109-90-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H361 |
| Precautionary statements | P280-P301+P312+P330 |
| Hazard Codes | Xn,T,F |
| Risk Statements | 22-63-39/23/24/25-23/24/25-11 |
| Safety Statements | 22-36/37/39-45-36/37-16-7 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | DE8750000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 2933910000 |
| Toxicity | LD50 in mice (mg/kg): 700 orally; 290 i.p.; LD50 orally in rats: >1000 mg/kg (Brunaud) |
CLORAZEPATE DIPOTASSIUM SALT Chemical Properties,Uses,Production
Chemical Properties
Pale Yellow Solid
Originator
Tranxene,Clin-Comar-Byla,France,1968
Uses
Anxiolytic. Controlled substance (depressant).
Definition
ChEBI: The compound of monopotassium clorazepate with potassium hydroxide. It is used for the management of anxiety disorders, for the short-term relief of anxiety, as adjunctive therapy in the management of epilepsy, and for the symptomatic relief of acute alcoh l withdrawal.
Manufacturing Process
(A) Preparation of (2-Amino-5-Chlorophenyl)Phenylmethaneimine (4356 CB):
A solution of 228.7 g (1.5 mold of 2-amino-5-chlorobenzonitrilein 1,800 ml of
dry ether is added slowly in the course of about 3.5 hours to a solution of
phenyl magnesium bromide prepared from 109 g (4.5 g-atoms) of magnesium
turnings and 848 g (5.4 mols) of bromobenzene in 3,600 ml of anhydrous
ether, and the mixture then heated under reflux for 15 hours.
The complex is decomposed by stirring the reaction mixture into a solution
prepared from 500 g of ammonium chloride in 2,000 mi of water to which 3
kg of crushed ice have been added. After extraction and washing, the ether is
evaporated in vacuo at 40°C. The oily residue is taken up in 500 ml of
petroleum ether and left to crystallize by cooling at -20°C. The yellowish
crystals formed are dried (309 g); MPk (Kofler block): 74°C; yield: 92%.
(B) Preparation of 7-Chloro-3-Methoxycarbonyl-5-Phenyl-2-Oxo-2,3-Dihydro-
1H-Benzo[f]-1,4-Diazepine (4347 CB): A solution of 9.2 g (0.04 mol) of
compound 4356 CB in 20 ml of methanol is added dropwise, in the course of
one hour and 30 minutes, to a boiling solution of 9.2 g (0.05 mol) of the
hydrochloride of methyl aminomalonate in 30 ml of methanol. When this is
completed, heating under reflux is continued for 30 minutes and the product
then concentrated to dryness under reduced pressure. The residue is taken up
in water and ether, the ethereal layer separated, the product washed with
water and dried over sodium sulfate. The solvent is evaporated under reduced
pressure. The residue, which consists of the methyl ester, could not be
obtained in the crystalline state. It is dissolved in 25 ml of acetic acid, heated
under reflux for 15 minutes, the product evaporated to dryness and the
residual oil taken up in ether. A colorless solid separates which is filtered by
suction and recrystallized from methanol. Colorless crystals are obtained (4.7
9); MPk (Kofler block): 226°C. A second crop (1.5 g) is obtained on
concentration of the mother liquor; MPk (Kofler block): 222°C; total quantity
6.2 g, corresponding to a yield of 47%.
(C) Preparation of Dipotassium Salt of [2-Phenyl-2-(2-Amino-5-Chlorophenyl)-
1-Azavinyl] Malonic Acid (4306 CB): 50 g of caustic potash are dissolved in
1,350 ml of 96% ethyl alcohol, and 82 g (0.25 mol) of compound 4347 CB are
then added all at once at a temperature of about 70°C. The solid dissolves
rapidly to form a yellow solution which then loses color while simultaneously
an abundant colorless precipitate appears.
After cooling, the solid is filtered by suction and washed with alcohol at 96°C.
The product is dried at ordinary temperature in a high vacuum. A colorless
solid is obtained (quantitative yield), which is completely soluble in water. The
aqueous solution is strongly alkaline in reaction; when acidified with acetic
acid and heated on a water bath, it yields a precipitate of 7-chloro-5-phenyl-
2-oxo-2,3-dihydro-1H-benzo[f]-1,4-diazepine.
brand name
Tranxene (Ovation).
Therapeutic Function
Tranquilizer
General Description
Clorazepate dipotassium,7-chloro-2,3-dihydro-2-oxo-5-phenyl-1H-1,4-benzodiazepine-3-carboxylic acid dipotassium salt monohydrate(Tranxene), can be considered a prodrug. Inactive itself, itundergoes rapid decarboxylation by the acidity of thestomach to nordazepam (a major active metabolite of diazepam),which has a long half-life and undergoes hepaticconversion to active oxazepam. Despite the polar characterof the drug as administered, because it is quickly convertedin the GI tract to an active nonpolar compound, it has aquick onset, overall long half-life, and shares similar clinicaland pharmacokinetic properties to chlordiazepoxideand diazepam.
CLORAZEPATE DIPOTASSIUM SALT Preparation Products And Raw materials
57109-90-7(CLORAZEPATE DIPOTASSIUM SALT)Related Search:
1of4