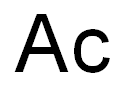Actinium
- CAS No.
- 7440-34-8
- Chemical Name:
- Actinium
- Synonyms
- actinium atom
- CBNumber:
- CB41366277
- Molecular Formula:
- C2H4O
- Molecular Weight:
- 44.05
- MDL Number:
- MOL File:
- 7440-34-8.mol
| Melting point | 1050 ±50° |
|---|---|
| Boiling point | bp ~3300° |
| Density | d25 10.07 |
| form | silvery metal |
| color | silvery metal; cubic |
| FDA UNII | NIK1K0956U |
Actinium Chemical Properties,Uses,Production
Description
Actinium-227 occurs in uranium ore and is a decay product of uranium-235. It is found in equilibrium with its decay products. It is prepared by bombarding radium atoms with neutrons. Chemically, the metal is produced by reducing actinium fluoride with lithium vapor at 1,100℃ to 1,300℃.
AcF3+Li→Ac + 3LiF
The element was discovered independently by A. Debierne and F. Giesel in 1899 and 1902, respectively. It is used in nuclear reactors as a source of neutrons.
Chemical Properties
Silvery metal; cubic crystal; melts at 1,051°C; vaporizes at 3,198°C; density 10.0 g/cm3
Physical properties
Silvery metal; cubic crystal; melts at 1,051°C; vaporizes at 3,198°C; density 10.0 g/cm3.
Occurrence
Actinium-227 occurs in uranium ore and is a decay product of uranium-235. It is found in equilibrium with its decay products. It is prepared by bombarding radium atoms with neutrons. Chemically, the metal is produced by reducing actinium fluoride with lithium vapor at 1,100°C to 1,300°C.
AcF3+Li→Ac + 3LiF
The element was discovered independently by A. Debierne and F. Giesel in 1899 and 1902, respectively. It is used in nuclear reactors as a source of neutrons.
History
The name Actinium derives from the Greek, aktis or akinis for “beam or ray” because in equilibrium with its decay products, actinium is a powerful source of alpha radiation. The discovery has been credited to the French chemist Andre-Louis Debierne in 1899. It was independently discovered by German chemist Friedrich Oskar Giesel in 1902, who called it emanium. It is thought that Debierne’s original preparation actually consisted of two thorium isotopes, 227Th and 230Th, but there was confusion in those early discoveries in radioactivity and Debierne’s claim prevailed and his name of actinium has been retained to this day. The longest half-life associated with this unstable element is 21.77 year 227Ac.
Definition
ChEBI: Actinium atom is a scandium group element atom, an actinoid atom and a f-block element atom.
Reactions
Actinium behaves like lanthanum forming mostly the trivalent salts of the metal. It is strongly electropositive, the first ionization potential being 5.17eV. Reacts with HCl forming AcCl3; also reacts with organic acids forming corresponding salts; combustion in air can produce oxide and nitride; susceptible to react with CO2 forming carbonate.
Hazard
Toxicity: Exposure to radiation can cause cancer.
Actinium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |