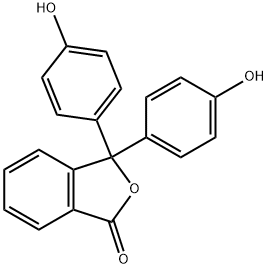
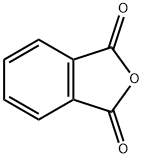
Phenolphthalein
- CAS No.
- 77-09-8
- Chemical Name:
- Phenolphthalein
- Synonyms
- PHENOLPHTALEIN;Fenolftalein;phenolphtaleine;PHENOLPHTHALEIN INDICATOR SOLUTION;3,3-bis(4-hydroxyphenyl)isobenzofuran-1(3H)-one;colax;phthalin;alpha-di(p-hydroxyphenyl)phthalide;2-[Bis(4-hydroxyphenyl)methyl]benzoic acid;.alpha, alpha.-Di(p-hydroxyphenyl)phthalide
- CBNumber:
- CB4141228
- Molecular Formula:
- C20H14O4
- Molecular Weight:
- 318.32
- MDL Number:
- MFCD00005913
- MOL File:
- 77-09-8.mol
- MSDS File:
- SDS
| Melting point | 261-263 °C (lit.) |
|---|---|
| Boiling point | 417.49°C (rough estimate) |
| Density | 1.27 g/cm3 at 32 °C |
| refractive index | 1.5400 (estimate) |
| Flash point | 24 °C |
| storage temp. | no restrictions. |
| solubility | Soluble in alcohol. Slightly soluble in ether. Slightly soluble in dimethyl sulfoxide and insoluble in benzene or hexane. |
| form | Solid |
| pka | 9.4(at 25℃) |
| Colour Index | 764 |
| color | White to yellow-white |
| PH Range | 8.0(colorless)-10(Red) |
| PH | 7.8~10.0 |
| Odor | Odorless |
| Water Solubility | <0.1 g/100 mL |
| λmax | 552nm, 553nm, 374nm, 205nm, 229nm, 276nm |
| Merck | 14,7243 |
| BRN | 284423 |
| Stability | Stable. Incompatible with strong oxidizing agents, alkalies. |
| Major Application | Display device, sensors, semiconductors, fuel cells, photoreceptors, electronic packaging, authentication system for secure documents, inks, correction fluid, paints, detection of defects in films, floor coatings, textiles, corrosion testing, explosive, concrete, toys, detecting lipase activity of crop seeds, soaps, method for prevention of drugmisuse, cosmetics, diapers, detecting viable cells, carbohydrates, antimalarial, treating amyloid-associated diseases |
| LogP | 2.410 |
| FDA 21 CFR | 310.545 |
| CAS DataBase Reference | 77-09-8(CAS DataBase Reference) |
| EWG's Food Scores | 6-9 |
| FDA UNII | 6QK969R2IF |
| Proposition 65 List | Phenolphthalein |
| ATC code | A06AB04 |
| NIST Chemistry Reference | Phenolphthalein(77-09-8) |
| IARC | 2B (Vol. 76) 2000 |
| EPA Substance Registry System | 3,3-Bis(4-hydroxyphenyl)phthalide (77-09-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H341-H350-H361f | |||||||||
| Precautionary statements | P202-P264-P280-P302+P352-P308+P313-P332+P313 | |||||||||
| Hazard Codes | Xn,T,F,Xi | |||||||||
| Risk Statements | 40-22-10-36/38-23/25-11-36/37/38-68-62-45-39/23/24/25-23/24/25 | |||||||||
| Safety Statements | 45-36/37-33-24-16-7-36-26-53 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | SM8380000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29322910 | |||||||||
| NFPA 704 |
|
Phenolphthalein price More Price(49)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 105945 | Phenolphthalein ACS reagent | 77-09-8 | 100g | $57.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 105945 | Phenolphthalein ACS reagent | 77-09-8 | 500g | $191 | 2024-03-01 | Buy |
| Sigma-Aldrich | 319236 | Phenolphthalein solution 0.5?wt. % in ethanol: water (1:1) | 77-09-8 | 100ML | $28.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 105945 | Phenolphthalein ACS reagent | 77-09-8 | 2.5kg | $641 | 2024-03-01 | Buy |
| TCI Chemical | P0094 | Phenolphthalein >98.0%(T) | 77-09-8 | 25g | $20 | 2024-03-01 | Buy |
Phenolphthalein Chemical Properties,Uses,Production
acid–base indicator
Phenolphthalein is another weak organic acid. It is not particularly water soluble, so we generally dissolve it in aqueous ethanol. The ethanol explains the pleasant, sweet smell of phenolphthalein solutions.
Phenolphthalein is colourless and clear in acidic solutions, but imparts an intense puce pink colour in alkaline solutions of higher pH, with λ(max) = 552 nm. The coloured form of phenolphthalein contains a quinone moiety; in fact, any chromophore based on a quinone has a red colour. But if a solution is prepared at pH 7 (e.g. as determined with a pH meter), we find the phenolphthalein indicator is still colourless, and the pink colour only appears when the pH reaches 8.2. Therefore, we have a problem: the indicator has not detected neutrality, since it changes colour at too high a value of pH. in fact, only a tiny incremental addition of alkali solution is needed to substantially increase the solution pH by several pH units. In other words, a fraction of a drop of alkali solution is the only difference between pH 7 (at the true volume at neutralization) and pH 8 when the phenolphthalein changes from colourless to puce pink.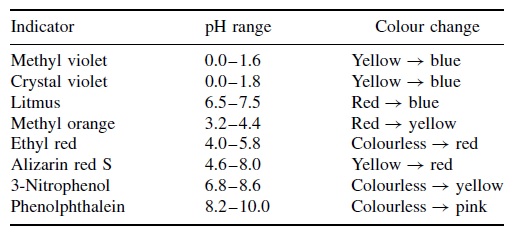
Figure 1 lists the pH changes for a series of common pH indicators. The colour changes occur over a wide range of pHs, the exact value depending on the indicator chosen. Methyl violet changes from yellow to blue as the pH increases between 0 and 1.6. At the opposite extreme, phenolphthalein responds to pH changes in the range 8.2 to 10.
Chemical Properties
Phenolphthalein is a weak organic acid, at room temperature, appearing as white or microstrip yellow small crystals, being odorless, tasteless, insoluble in water and soluble in alcohol (ethanol) and ether. When dissolved in alcohol solution, it can be made of acid and alkali indicator. It is colorless in acid solution while exhibiting red color in the carbonate solution of alkali metal. However, if placed in the concentrated alkali solution, it will produce colorless tri-metal Salt so that the red color will fade. In view of this feature, laboratory commonly uses it as a kind of acid and alkali titration indicator with the discoloration range of PH being around 8.2 to 10.0, turning from colorless to red. When the phenolphthalein reagent is dropped into water or neutral, acidic aqueous solution, there will be white turbidity. This is due to that the alcohol is easily soluble in water, so that the reagent water-insoluble phenolphthalein is precipitated out. The other major applications are as follows:
1, the pharmaceutical raw materials in pharmaceutical industry: it is suitable for habitual stubborn constipation, including tablets, suppositories and other formulations.
2, it can be used for organic synthesis: mainly used in synthesizing plastics, especially in synthesizing the phthalazinone poly aryl ether ketone poly aryl ether ketone polymers. This kind of polymers, because of its excellent heat resistance, water resistance, corrosion resistance, heat aging resistance and good processability, fibers, coatings and composites made of it are widely used in the fields of electrical and electronic equipment, transportation and aerospace, atomic energy engineering and military.
3, used as acid and alkali indicator, non-aqueous solution titration indicator and chromatography analysis reagents.
General Description
Phenolphthalein mainly acts on the large intestine, which produces semi-liquid excreta in 4 to 8 hours with little or no colic. The claim that yellow phenolphthalein is three times stronger than white remains unproven. As a result of enterohepatic circulation, the effect of a single dose is sustainable for 3 to 4 days. The drug is an active member of many laxatives that can be sold legally without prescription.
Serious adverse effects are rare but can occur when used in excess. Phenolphthalein should be avoided on the elderly, because its long-term role can cause severe depletion of water and electrolytes. Dermatitis (fixed rash, itching, burning sensation, blistering, and residual pigmentation) can occur in allergic patients. There were reports of a fatal anaphylaxis but no definite causative relationship with phenolphthalein. There are occasional reports of non-thrombocytopenic purpura. Long-lasting administration can occasionally caused dehydration and electrolyte imbalance due to excessive diarrhea. Phenolphthalein causes alkaline urine or faeces pink.
Phenolphthalein molecular structure
This product can be obtained by mixing phthalic anhydride with phenol, followed by co-heating with sulfuric acid.
Phenolphthalein has its chemical name be 2- [bis- (4-hydroxyphenyl) methyl] benzoic acid. Molecular formula: C20H16O4, relative molecular mass: 318.33. The structural formula is shown in Figure 1 (in fact, it is often existed in the form of colorless lactone, see Figure 2). It appears as triclinic white to yellowish crystalline powder with the melting point of 237 ~ 259 ℃ and the relative density of 1.277 (20/4 ℃). It is easily soluble in ethanol, ether and other organic solvents, slightly soluble in water. It has a weak acidity and is almost completely presented in the molecule state even in very dilute solution. It is soluble in alkaline solution, exhibiting red color in alkaline solution, but is colorless in acidic solution. The three benzene rings in the phenolphthalein molecule are linked to the carbon atoms of a sp3 hybrid center, and there is no conjugate relationship between the benzene rings, being colorless. After coming across alkali, the lactone ring open and generate disodium salt, the central carbon atoms are transferred into the sp2 hybrid state, containing benzoquinone structure. At this time, it forms conjugated system with the three benzene rings which are red; but in excess of alkali, but it is further converted to sp3 hybrid state so that the conjugate system disappears, the color also will recede.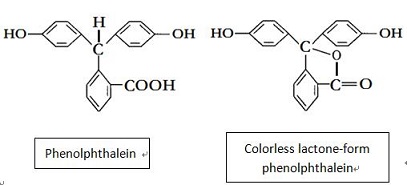
Phenolphthalein indicator
Phenolphthalein indicator is a commonly used acid/alkali titration indicator in analytic chemistry. Phenolphthalein, in alkaline solution, has its colorless lactone ring open to form quinone structure with the color exhibiting red. Upon coming across strong alkali, phenolphthalein can be converted into colorless carboxylate. Its color range is pH 7.0 (colorless) ~ 10.0 (red). The test solution was prepared by dissolving 1 g of 90% ethanol per liter of ethanol.
Phenolphthalein is colorless in the solution of pH below 8.5, become red at pH higher than 9. Upon excess amount of alkali and no color, the color process is as follows: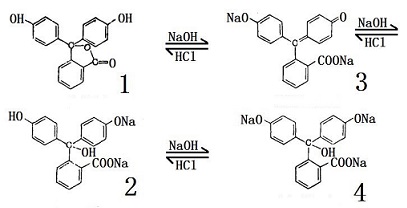
Phenolphthalein, when used as acid-alkali titration indicator, is generally used at a concentration of 1% concentration. 1 g of phenolphthalein was dissolved in 100 ml of 80% alcohol; slowly drop 0.1 mol / l sodium hydroxide to reddish (for neutralizing the small amount of acid potentially existing in alcohol). The pH range of discoloration is 8.2 to 10. It is colorless upon PH <8.2, is red upon pH> 10. The principle of color change is mainly due to that under different acid and alkali conditions, the structure of the molecule itself changes and further causes different colors. Mixing the phenolphthalein and other indicators can formulate the widely used indicator (also known as a general indicator).
Mix 1.3 grams of phenolphthalein, 0.9 grams of bromo-thymol blue, 0.4 grams of methyl red and 0.2 grams of thymol blue and dissolve in 1 liter 70% to 80% alcohol. After completely dissolving, then add some 0.1 mol / l sodium hydroxide makes it become just green before application. The advantage is that the color change with the pH changes is remarkable: red for pH = 4; orange for pH 5; yellow for pH 6, green for pH 7; cyan for pH 8; blue for pH 9; and purple for pH 10.
Different broad indicator can be formulated as needed. Phenolphthalein indicator is not only used in laboratories, but also widely used in industrial and agricultural production. In addition to being used in the pH control of the reaction and acid/alkali titration analysis during the manufacturing of chemical products, it is also used for determining the pH of the weaving in the printing and dyeing industry. This is very important for printing and dyeing processing. For example, if the mercerized cloth contains residual alkali that has not been washed, the dyeing will have no shine. However, the phenolphthalein indicator alone can only determine whether the texture of the weave is acidic or alkaline and its pH range, and can’t further determine its specific pH. The wide range of indicators used above can be clearly reflected of the acid and alkali strength in the cloth. Phenolphthalein, in medicine can also be used as laxative, because it can stimulate the peristalsis of the intestinal to promote defecation. However, it can occasionally cause allergic reactions such as dermatitis, thus should be used with attention.
Phenolphthalein test paper
Phenolphthalein test paper is one of the simple test papers commonly used in chemical analysis. It’s highly targeted. Its turns red in case of alkali, and is colorless in acid and neutral solution. To test the presence of ammonia in the air, it will be appears light red. The preparation method is simple: the filter paper is immersed in phenolphthalein solution and taken out for drying after full absorption.
Pharmacology and Toxicology
Phenolphthalein can be used for the treatment of habitual refractory constipation, mainly acting on the colon. After oral administration, it can be subject to slowly decomposition under the effect of the small intestinal alkaline fluid to form soluble sodium salt, thereby stimulating the plexus inside the intestinal wall. It directly acts on the intestinal smooth muscle and increase peristalsis, while also inhibiting the absorption of water within the intestine, so that water and electrolyte accumulates in the colon, resulting in laxative effect. It has mild effect and rarely causes intestinal cramps.
Pharmacokinetics
About 15% is absorbed after oral administration. The absorbed drugs mainly exist in the form of glucuronide to be excreted from urine or the feces, some can also be excreted to the intestine through the bile, reabsorbed in the intestine, to form intestinal - liver circulation and prolong the role time. At 4 to 8 hours after the drug administration, the patients will discharge soft stool. The excretion time for drug administration once needs 3 to 4 days. This product can also be secreted from milk.
Adverse reactions
Phenolphthalein mainly takes effect in the large intestine, leading to the formation of little amount of semi-liquid feces in 4 to 8 hours without colic. The claim regarding that the yellow phenolphthalein is three times stronger than the white version has not been proven. As a result of enterohepatic circulation, the effect of a single dose can last 3 to 4 days. This drug is an active member in a lot of laxative preparations that can be legally sold without a doctor's prescription.
Severe adverse effects are rare but can occur in excess amount. For the elderly, phenolphthalein should be hanged, because its lasting effect can cause serious depletion of water and electrolytes. In allergic patients, it can cause occur dermatitis (fixed rash, itching, burning sensation, blistering, and residual pigmentation). There are reports of fatal allergic reactions, but have not yet determined to be related with phenolphthalein. There are occasional reports of non-thrombocytopenic purpura and the occurrence of dehydration and electrolyte imbalance caused excessive diarrhea after long-term application. Phenolphthalein makes alkaline urine or feces turn pink.
Application
This product is a commonly used acid indicator with the range of color change between pH 8.2 (colorless) to 10.0 (red). Phenolphthalein is also a drug, can be used as a laxative, acting on the colon for the treatment of constipation.
Used as an acid-base indicator; as irritant laxative for the treatment of constipation
Production method
It is derived from the condensation of phthalic anhydride and phenol.
Chemical Properties
white to light yellow crystal powde
Uses
Phenolphthalein is one of those chemicals that is commonly used in the chemistry laboratory to tell if a solution is acidic or alkaline . These chemicals are called acid - base indicators and used as indicator for acidimetric titrations. Phenolphthalein exerts laxative effects by stimulating the intestinal mucosa and constricting smooth muscles. However, phenolphthalein is no longer used as a laxative due to the suspected carcinogenicity of this compound.
Uses
Phenolphthalein can be used as an inhibitor and pH indicator. It also induces centrosome amplification and tubulin depolymerization in vitro.
Definition
An acid–base indicator that is colorless in acid solutions and becomes red if the pH rises above the transition range of 8–9.6. It is used as the indicator in titrations for which the end point lies clearly on the basic side (pH>7), e.g. oxalic acid or potassium hydrogentartrate against caustic soda.
Definition
phenolphthalein: A dye used as anacid-base indicator. It is colourlessbelow pH 8 and red above pH 9.6. Itis used in titrations involving weakacids and strong bases. It is also usedas a laxative.
brand name
Evac-Q-Tabs (Savage); Ex-Lax (Novartis); Modane (Savage); Prulet (Mission Pharmacal);Agaffin;Alophen pills;Anodyne dellipsoids 4;Ap-la-day;Bold laxine;Bom-bon;Bon-bon;Canisan;Certolax;D & m tablets;Darmol;Euchessinia;Evac-qwik tablets;Evactil;Feen-a-mint;Fractines vichy;Fractine-vichy;Fructines-vichy;Kalimalterin;Kest;Kondremul with phenolphtalein;Laxante yer;Laxatabs;Laxatone;Laxen busto;Minilax;Modane plus;Mucinum;Musilaks;Neoprunex;Neopurghes;Novopuren;Paradeines;Peplax;Petrolaglar emulsion;Petro-mul-phen;Phillips laxcaps;Prifunal;Prunetta;Pugrante el aleman;Purganol;Purganos-daguin;Purgant aleman;Purgante orravan;Purgenum;Purgestol;Purgoids;Purgyl;Purjen sahap;Sarolax;Thalinol mrt;Unisvelt;Veracolate.
World Health Organization (WHO)
Phenolphthalein has been widely used as a laxative since its cathartic activity was first described in 1902. Because it undergoes enterohepatic circulation it is eliminated slowly and it has been associated with adverse effects, notably skin reactions, potassium loss and atonia. This has led to the withdrawal of phenolphthalein from pharmaceutical preparations in several countries. Elsewhere, it remains available, often in over-the-counter preparations.
General Description
Phenolphthalein solution is a synthetic indicator. It is colorless in acidic and neutral conditions. Phenolphthalein gives pink color when added to a base, therefore it is considered as a base indicator.
Safety Profile
Confirmed carcinogen. US Food and Drug Administration recommends removal from laxative formulations. Moderately toxic by intraperitoneal route. Human systemic effects: changes in urine composition, gastritis, nausea or vomiting. Used in medicine as a laxative; in chemistry as an indicator. When heated to decomposition it emits acrid smoke and irritating fumes
Carcinogenicity
Phenolphthalein is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Purification Methods
Dissolve it in EtOH (7mL/g), then dilute it with eight volumes of cold water, filter and heat on a water-bath to remove most of the alcohol and the phenolphthalein that precipitates is filtered off and dried in vacuo.[Beilstein 18 II 119, 18 III/1V 1945, 18/4 V 188.]
Phenolphthalein Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Aumei New Material Co., Ltd. | +86-027-87366298 +8615927270571 | tianyi@skbiology.cn | China | 294 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 255 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Wuhan Xinhao Biotechnology Co., Ltd | +86-18120578002 +86-18120578002 | xinhao-6@xinhaoshengwu.com | China | 350 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 951 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 1696 | 58 |
Related articles
- Phenolphthalein Discoloration in Chemistry
- Phenolphthalein, an organic phthalein compound widely used as an acid-base indicator. As an indicator of the pH of a solution,....
- Jan 4,2023
- Toxic Effects of Phenolphthalein
- Phenolphthalein is a chemical compound, often used as an indicator in titrations and its formula is C20H14O4. It is a weak aci....
- Nov 20,2019
View Lastest Price from Phenolphthalein manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Phenolphthalein
77-09-8
|
US $5.00 / kg | 10kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-22 | Phenolphthalein
77-09-8
|
US $50.00 / kg | 1kg | 99.10% | 50000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-22 | Phenolphthalein
77-09-8
|
US $0.00-0.00 / Kg/Bag | 2Kg/Bag | 0.99 | 20 tons | Sinoway Industrial co., ltd. |
-

- Phenolphthalein
77-09-8
- US $5.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Phenolphthalein
77-09-8
- US $50.00 / kg
- 99.10%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- Phenolphthalein
77-09-8
- US $0.00-0.00 / Kg/Bag
- 0.99
- Sinoway Industrial co., ltd.