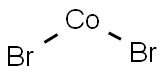
COBALT(II) BROMIDE
- CAS No.
- 7789-43-7
- Chemical Name:
- COBALT(II) BROMIDE
- Synonyms
- CoBr2;COBALT BROMIDE;dibromocobalt;cobaltdibromide;COBALTOUS BROMIDE;COBALT(+2)BROMIDE;Cobalt(Ⅱ) Bromide;COBALT(II) BROMIDE;Cobalt(ll) bromide;Mancobride mancanese
- CBNumber:
- CB4144320
- Molecular Formula:
- Br2Co
Lewis structure
- Molecular Weight:
- 218.74
- MDL Number:
- MFCD00016017
- MOL File:
- 7789-43-7.mol
- MSDS File:
- SDS
| Melting point | 678°C |
|---|---|
| Density | 4.909 g/mL at 25 °C(lit.) |
| solubility | acetone: soluble(lit.) |
| form | beads |
| color | Green |
| Specific Gravity | 4.909 |
| Water Solubility | Soluble in water. Soluble in methyl acetate, ether, alcohol, acetone. |
| Sensitive | Hygroscopic |
| Merck | 14,2435 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| CAS DataBase Reference | 7789-43-7(CAS DataBase Reference) |
| FDA UNII | 7M7RX75BAL |
| EPA Substance Registry System | Cobalt bromide (7789-43-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H317-H334-H341-H350-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P301+P312-P302+P352-P308+P313 | |||||||||
| Hazard Codes | T,Xn | |||||||||
| Risk Statements | 45-20/21/22-36/37/38-42/43 | |||||||||
| Safety Statements | 36-45-36/37/39-26-22-53 | |||||||||
| RIDADR | UN 3077 9 / PGIII | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GF9595000 | |||||||||
| TSCA | Yes | |||||||||
| NFPA 704 |
|
COBALT(II) BROMIDE price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 334022 | Cobalt(II) bromide 99% | 7789-43-7 | 50g | $83.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 334022 | Cobalt(II) bromide 99% | 7789-43-7 | 250g | $332 | 2024-03-01 | Buy |
| Alfa Aesar | 023149 | Cobalt(II) bromide, anhydrous, 97% | 7789-43-7 | 50g | $89.2 | 2024-03-01 | Buy |
| Alfa Aesar | 023149 | Cobalt(II) bromide, anhydrous, 97% | 7789-43-7 | 250g | $390 | 2024-03-01 | Buy |
| Sigma-Aldrich | 427136 | Cobalt(II) bromide anhydrous, beads, 10 mesh, 99.99% trace metals basis | 7789-43-7 | 1g | $102 | 2024-03-01 | Buy |
COBALT(II) BROMIDE Chemical Properties,Uses,Production
Chemical Properties
The green anhydrous salt is prepared by dehydration of the red hexahydrate or by the action of bromine on heated cobalt. It is very soluble in water and soluble in many polar organic solvents; it deliquesces in moist air to a red solution. The hexahydrate crystallizes from aqueous solution at room temperature; it melts at 100°, evolving water and forming the purple dihydrate.
Chemical Properties
Reddish violet crystalline powder
Uses
Cobalt(II) bromide is used as a catalyst in organic synthesis. It is used as a precursor in the production of ethylsulfanyl)porphyrazinato)cobalt(II), which gives the possibility of intermolecular ferromagnetic interactions.
Uses
Used in the preparation of a new complex, Co(OESPz), which offers the possibility of intermolecular ferromagnetic interactions.1
General Description
COBALT(II) BROMIDE is a red violet crystalline solid. COBALT(II) BROMIDE is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. COBALT(II) BROMIDE is used as a catalyst in the production of other chemicals.
Air & Water Reactions
Water soluble.
Reactivity Profile
Acidic salts, such as COBALT(II) BROMIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. When heated to decomposition can give off highly toxic fumes of Br [USCG, 1999].
Health Hazard
SOLID: Irritating to skin and eyes. Harmful if swallowed.
Fire Hazard
Not flammable. POISONOUS FUMES ARE PRODUCED WHEN HEATED TO DECOMPOSITION. When heated to decomposition can give off highly toxic fumes of Br.
Purification Methods
Crystallise it from water (1mL/g) by partial evaporation in a desiccator. The anhydrous salt is soluble in EtOH, Me2CO, MeOAc to form blue-coloured solutions. [Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1517 1965.]
COBALT(II) BROMIDE Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9705 | 58 |
| Henan Alfa Chemical Co., Ltd | +8618339805032 | alfa4@alfachem.cn | China | 12757 | 58 |
7789-43-7(COBALT(II) BROMIDE)Related Search:
1of4