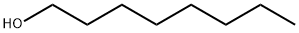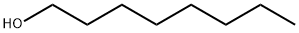1-Octanol
- CAS No.
- 111-87-5
- Chemical Name:
- 1-Octanol
- Synonyms
- OCTANOL;N-OCTANOL;caprylic;OCTAN-1-OL;OCTYL ALCOHOL;N-OCTYL ALCOHOL;1-Octano;N-CAPRYLIC ALCOHOL;Octilin;n-Octano
- CBNumber:
- CB4157940
- Molecular Formula:
- C8H18O
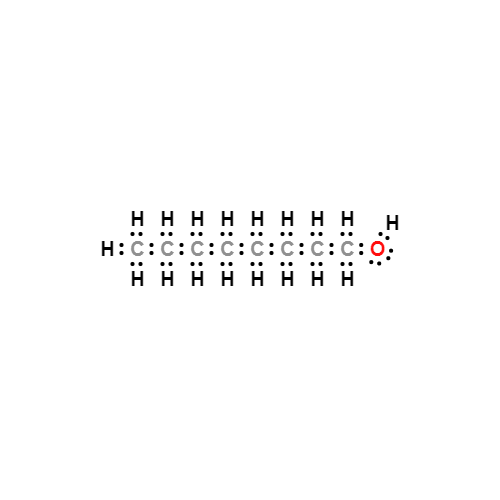
Lewis structure
- Molecular Weight:
- 130.23
- MDL Number:
- MFCD00002988
- MOL File:
- 111-87-5.mol
- MSDS File:
- SDS
| Melting point | −15 °C(lit.) |
|---|---|
| Boiling point | 196 °C(lit.) |
| Density | 0.827 g/mL at 25 °C(lit.) |
| vapor density | 4.5 (vs air) |
| vapor pressure | 0.14 mm Hg ( 25 °C) |
| refractive index |
n |
| FEMA | 2800 | 1-OCTANOL |
| Flash point | 178 °F |
| storage temp. | Store below +30°C. |
| solubility | water: partially soluble107g/L at 23°C |
| pka | 15.27±0.10(Predicted) |
| form | liquid |
| color | APHA: ≤10 |
| Relative polarity | 0.537 |
| Odor | Alcohol like |
| Odor Threshold | 0.0027ppm |
| explosive limit | 0.8%(V) |
| Odor Type | waxy |
| Evaporation Rate | 0.007 |
| Viscosity | 5.58mm2/s |
| Water Solubility | insoluble |
| λmax |
λ: 215 nm Amax: 1.00 λ: 225 nm Amax: 0.50 λ: 235 nm Amax: 0.20 λ: 250 nm Amax: 0.05 λ: 300-400 nm Amax: 0.01 |
| JECFA Number | 97 |
| Merck | 14,6751 |
| BRN | 1697461 |
| Dielectric constant | 10.3(20℃) |
| Stability | Stable. Flammable. Incompatible with strong oxidizing agents. |
| InChIKey | KBPLFHHGFOOTCA-UHFFFAOYSA-N |
| LogP | 3.5 at 23℃ |
| Substances Added to Food (formerly EAFUS) | 1-OCTANOL |
| FDA 21 CFR | 177.1200 |
| CAS DataBase Reference | 111-87-5(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | NV1779205D |
| NIST Chemistry Reference | 1-Octanol(111-87-5) |
| EPA Substance Registry System | 1-Octanol (111-87-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H412 | |||||||||
| Precautionary statements | P264-P273-P280-P305+P351+P338-P337+P313-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/38 | |||||||||
| Safety Statements | 26-36/37-37/39 | |||||||||
| RIDADR | 3082 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | RH6550000 | |||||||||
| Autoignition Temperature | 523 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29051680 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rabbit > 2000 mg/kg | |||||||||
| NFPA 704 |
|
1-Octanol price More Price(67)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W280011 | 1-Octanol natural, ≥98%, FCC | 111-87-5 | 1kg | $128 | 2024-03-01 | Buy |
| Sigma-Aldrich | W280011 | 1-Octanol natural, ≥98%, FCC | 111-87-5 | 4kg | $402 | 2024-03-01 | Buy |
| Sigma-Aldrich | W280003 | 1-Octanol ≥98%, FCC, FG | 111-87-5 | 1kg | $76.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W280003 | 1-Octanol ≥98%, FCC, FG | 111-87-5 | 4kg | $220 | 2024-03-01 | Buy |
| Sigma-Aldrich | W280003 | 1-Octanol ≥98%, FCC, FG | 111-87-5 | 20kg | $362 | 2024-03-01 | Buy |
1-Octanol Chemical Properties,Uses,Production
Description
1-Octanol (111-87-5) is a colorless and transparent liquid with the chemical formula C8H18O. It is a fatty alcohol consisting of a linear, saturated chain of eight carbon atoms. Although 1-Octanol is not highly soluble in water, it can dissolve easily in alcohol, ether, and chloroform. This compound is commonly used as a raw material in the production of fragrances, octanal, octanoic acid, and its esters. It also functions as a solvent, defoamer, and lubricating oil additive. Notably, 1-Octanol poses a low level of toxicity, but it can be irritating to the skin and eyes. Nonetheless, it is considered safe to use under normal conditions because of its low vapor pressure.
Chemical Properties
1-Octanol has a fresh, orange-rose odor, quite sweet and an oily, sweet, slightly herbaceous taste.
Occurrence
Reported as frequently occurring in essential oils as an ester; the free alcohol has been reported found in the essential oils of green tea, grapefruit, California orange, Andropogon intermedius, Heracleum villosum, violet leaves, Anethum graveolens and bitter orange. Also reported in over 200 foods and beverages including apple, apricot, banana, citrus peel oils and juices, many berries, guava, grapes, melon, papaya, peach, pear, asparagus, kohlrabi, leek, peas, tomato, potato, clove, ginger, mustard, spearmint oil, wheat bread, many cheeses, cooked egg, butter, milk, fish, meats, beer, rum, whiskies, cognac, cider, sherry, grape wines, cocoa, tea, nuts, honey, soybean, coconut, passion fruit, olive, avocado, plum, rose apple, beans, mushroom, starfruit, Bantu beer, sesame seed, cauliflower, broccoli, tamarind, fruit brandies, fig, cardamom, coriander seed and leaf, rice, quince, litchi, dill, lovage, sweet corn, corn oil, malt, kiwifruit, loquat, Bourbon vanilla, clary sage, oysters, crayfish, clam and Chinese quince.
Uses
1-Octanol is used as a precursor to prepare esters, which is used in perfumes and flavorings. It is used as food additive, anti-foaming agent and emulsifier in anti-rust emulsions. It is widely involved in chemical industry for the synthesis of ethoxylates, alkyl sulfates and ether sulfates. It is used as a solvent in paints, varnishes, waxes, and surface coatings and mainly involved in agricultural chemistry to inhibit excessive growth of tobacco plants.
Definition
ChEBI: Octan-1-ol is an octanol carrying the hydroxy group at position 1. It has a role as a plant metabolite. It is an octanol and a primary alcohol.
Preparation
1-Octanol may be prepared by reduction of some caprylic esters such as methyl caprylate with sodium ethoxide.
Production Methods
1-Octanol is made commercially by sodium reduction or high-pressure catalytic hydrogenation of the esters of naturally occurring caprylic acid or by oligomerization of ethylene using aluminum alkyl technology.
Application
1-octanol is mainly used in the production of plasticizer, extractant and stabilizer, and as an intermediate of solvent and perfume. In the field of plasticizers, 1-octanol generally refers to 2-ethylhexanol, which is a bulk raw material of one million tons and is far more valuable than n-octanol in industry. Octanol itself is also used as a spice, blending roses, lilies and other flower fragrance as a soap flavor. This product is an edible spice that is allowed to be used according to Chinese GB2760-86. Mainly used to make coconut, pineapple, peach, chocolate and citrus flavors.
Aroma threshold values
Detection: 42 to 480 ppb
Taste threshold values
Taste characteristics at 2 ppm: waxy, green, citrus, orange and aldehydic with a fruity nuance.
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 38, p. 2097, 1990 DOI: 10.1248/cpb.38.2097
Tetrahedron Letters, 23, p. 157, 1982 DOI: 10.1016/S0040-4039(00)86773-8
General Description
A clear colorless liquid with a penetrating aromatic odor. Insoluble in water and floats on water. Vapors heavier than air. Vapors may irritate the eyes, nose, and respiratory system.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Attacks plastics [Handling Chemicals Safely 1980. p. 236]. Acetyl bromide reacts violently with alcohols or water [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: an explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73. 1967; J, Org. Chem. 28:1893. 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M. 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
Health Hazard
Irritates skin and eyes.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Blocks T-type Ca2+ channels
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. Mutation data reported. A skin irritant. Combustible liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use water foam, fog, alcohol foam, dry chemical, CO2. See also ALCOHOLS.
Synthesis
1-octanol exists in essential oils such as bitter orange, grapefruit, sweet orange, green tea and violet leaves in free state, or in acetate, butyrate and isovalerate. In industrial production, octanal can be reduced or prepared by using octanoic acid in coconut oil. It can also be prepared by carbonyl synthesis with heptene-1 as raw material. HEPTENE, carbon monoxide and hydrogen form aldehyde in the presence of cobalt salt at 150-170 ℃ and high pressure of 20-30Mpa. After cobalt removal, HEPTENE is hydrogenated to primary alcohol under pressure with nickel catalyst. This method has mature production technology abroad.
Carcinogenicity
There was no evidence of tumors
in the cancer screening lung adenoma study in which mice
were injected intraperitoneally with 100 and 500 mg/kg 1-
octanol three times a week for 8 weeks. This assay has
not been validated as a reliable screen for cancer.
1-Octanol is a weak skin tumor promoter when applied
three times a week for 60 weeks to mice skin that had been
initiated with dimethylbenz[a]anthracene.
Metabolism
The primary aliphatic alcohols undergo two general reactions in vivo, namely oxidation to carboxylic acids and direct conjugation with glucuronic acid. The first reaction proceeds with the intermediate formation of an aldehyde, and the carboxylic acid from this may be either oxidized completely to carbon dioxide or excreted as such or combined with glucuronic acid as an ester glucuronide. The extent to which an alcohol undergoes the second reaction, i.e. direct conjugation to an ether glucuronide, appears to depend upon the speed of the first reaction, for alcohols which are rapidly oxidized form very little ether glucuronide unless given in high doses (Williams, 1959).
Purification Methods
Fractionally distil it under reduced pressure. Dry it with sodium and again fractionally distil or reflux with boric anhydride and re-distilled (b 195-205o/5mm), the distillate being neutralised with NaOH and again fractionally distilled. Also purify it by distillation from Raney nickel and by preparative GLC. [Beilstein 1 IV 1756.]
1-Octanol Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2739 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7473 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 951 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 471 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 101 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
Related articles
- How to use 1-octanol correctly
- 1-octanol is an organic substance with the chemical formula of C8H18O. It is slightly soluble in water and soluble in alcohol,....
- Apr 13,2022
View Lastest Price from 1-Octanol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-19 | 1-Octanol
111-87-5
|
US $6.00 / KG | 1KG | More than 99% | 2000KG/MONTH | Hebei Saisier Technology Co., LTD | |
 |
2024-04-19 | Octanol
111-87-5
|
US $13.00 / L | 1L | 98% | 20T | Yujiang Chemical (Shandong) Co.,Ltd. | |
 |
2024-04-12 | octyl alcohol | US $50.00-30.00 / kg | 1kg | 99.8% | 500 | hebei hongtan Biotechnology Co., Ltd |
-

- 1-Octanol
111-87-5
- US $6.00 / KG
- More than 99%
- Hebei Saisier Technology Co., LTD
-

- Octanol
111-87-5
- US $13.00 / L
- 98%
- Yujiang Chemical (Shandong) Co.,Ltd.
-

- octyl alcohol
- US $50.00-30.00 / kg
- 99.8%
- hebei hongtan Biotechnology Co., Ltd