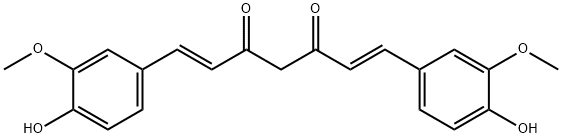
Curcumin
- CAS No.
- 458-37-7
- Chemical Name:
- Curcumin
- Synonyms
- TURMERIC EXTRACT;halad;haldar;curcuma;Diferuloylmethane;Curcumin, Natural Yellow 3, Diferuloylmethane;yo-kin;souchet;kurkumin;Crucumin
- CBNumber:
- CB4183426
- Molecular Formula:
- C21H20O6
- Molecular Weight:
- 368.38
- MDL Number:
- MFCD01868798
- MOL File:
- 458-37-7.mol
- MSDS File:
- SDS
| Melting point | 183 °C |
|---|---|
| Boiling point | 418.73°C (rough estimate) |
| Density | 0.93 |
| vapor density | 13 (vs air) |
| refractive index | 1.4155-1.4175 |
| Flash point | 208.9±23.6 °C |
| storage temp. | 2-8°C |
| solubility | ethanol: 10 mg/mL |
| form | powder |
| pka | 8.09(at 25℃) |
| Colour Index | 75300 |
| color | orange |
| Odor | Odorless |
| PH Range | Yellow (7.8) to red-brown (9.2) |
| Water Solubility | Slightly soluble (hot) |
| λmax | 430nm |
| Merck | 14,2673 |
| Solvent | Ethanol |
| Concentration | 1 mCi/ml |
| Specific Activity | 5-15 Ci/mmol |
| BRN | 2306965 |
| Stability | Stable, but may be light sensitive. Incompatible with strong oxidizing agents. |
| Major Application | Cosmetics, drug-eluting stents, inhibition of formation of skin-wrinkles, treating alzheimer’s disease, skin diseases, coronary restenosis, diabetes, obesity, leukemia, neurofibromas, cancer, antimicrobial, antiviral, antiinflammatory, antiprostate cancer |
| InChIKey | VFLDPWHFBUODDF-FCXRPNKRSA-N |
| LogP | 3.290 (est) |
| FDA 21 CFR | 310.545 |
| CAS DataBase Reference | 458-37-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | curcumin |
| FDA UNII | IT942ZTH98 |
| NCI Drug Dictionary | curcumin |
| EPA Substance Registry System | Curcumin (458-37-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MI5230000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29145000 | |||||||||
| Toxicity | LD50 Oral-Rat-12.200 mg/kg | |||||||||
| NFPA 704 |
|
Curcumin price More Price(102)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.20354 | Curcumin for synthesis | 458-37-7 | 2g | $58.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.20354 | Curcumin for synthesis | 458-37-7 | 10g | $79.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.20354 | Curcumin for synthesis | 458-37-7 | 50g | $285 | 2024-03-01 | Buy |
| Sigma-Aldrich | 08511 | Curcumin analytical standard | 458-37-7 | 10mg | $229 | 2024-03-01 | Buy |
| Sigma-Aldrich | 00280590 | Curcumin primary reference standard | 458-37-7 | 10mg | $123.2 | 2024-03-01 | Buy |
Curcumin Chemical Properties,Uses,Production
Food Additive
Curcumin has been widely used in the food industry as a common natural pigment for a long time. It is mainly used for the dyeing of canned food, sausage products and soy sauce products. The amount of curcumin used is determined by normal production needs. The product form of functional food with curcumin as the main component can be general food or some non-food forms, such as capsules, pills or tablets. For general food form, some yellow pigmented foods can be considered, such as cakes, sweets, beverages, etc.
Curcumin is a food additive approved by the Codex Alimentarius Commission of the Food and Agriculture Organization of the United Nations (FAO/WHO-1995). The newly promulgated "Standards for the Use of Food Additives" (GB2760-2011) stipulates that frozen drinks, cocoa products, chocolate and chocolate products and candies, gum-based candies, decorative candies, toppings and sweet sauces, batter, coating powder and frying powder , The maximum usage of curcumin in instant rice and noodle products, flavored syrup, compound seasoning, carbonated drinks and jelly is 0.15, 0.01, 0.7, 0.5, 0.3, 0.5, 0.5, 0.1, 0.01, 0.01 g/kg, respectively, margarine and its similar products, cooked nuts and seeds, fillings for grain products and puffed foods can be used in moderation according to production needs.
Description
Curcumin is the major yellow pigment in turmeric and curry and has antioxidant, anti-inflammatory, and antitumor activities. It inhibits nitric oxide (NO) production (IC50 = 6 μM) and reduces inducible nitric oxide synthase (iNOS) activity in LPS-stimulated RAW 264.7 cells. Curcumin inhibits release of histamine and the inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8 from HMC-1 mast cells. In vivo, curcumin decreases serum levels of histamine and TNF-α, inhibits histopathological changes of nasal mucosa, and decreases the number of sneezes and nasal rubbing in a mouse model of ovalbumin-induced rhinitis. Curcumin (100 or 200 mg/kg) prevents ovalbumin-induced accumulation of 3-nitrotyrosine (3-NT), a marker of oxidative stress, in mouse heart. Topical administration of curcumin (1-10 μmol) reduces the number of tumors induced by phorbol 12-myristate 13-acetate (TPA; ) in mouse skin. Dietary administration of curcumin reduces the number of tongue neoplasms and preneoplastic lesions induced by 4-nitroquinoline 1-oxide (4-NQO) in rats.
Description
The main source of curcumin is the root of Zingiberaceae Curcuma aromatica, rhizome of Curcuma longa (Jiang Huang), Curcuma zedoaria, and Acorus calamus. Among them, Jiang Huang contains about 3–6% curcumin. The traditional Chinese medicine, Jiang Huang, is the root tuber of perennial herbaceous plant Curcuma longa L. of family Zingiberaceae. It was firstly recorded in the “Tang materia medica” (Xin Xiu Ben Cao). It is pungent, bitter, and warm and enters the liver and spleen meridians. It activates the blood, moves qi, dredges meridians, and alleviates pain. In India and other Asian countries, Jiang Huang has more than 6000?years of application history. In Japan, Jiang Huang has a long history of health care, and the people of Okinawa Island regarded Jiang Huang as a holy tribute to the emperor. Jiang Huang mainly comes from Taiwan, Fujian, Guangdong, Guangxi, Yunnan, and Tibet of China and other regions in East Asia and Southeast Asia. It grows in warm and humid climate and sunny environment with abundant rainfall and fears cold frost, drought, and flood. At present, Chinese Pharmacopoeia only included Jiang Huang and Yu Jin which contains curcumin, while curcumin is not included.
Chemical Properties
Several species of Curcuma exist: C. xanthorrhyza, C. domestica, C. zedoafia, C. caesia and C. amada. Although all these are aromatic plants, C. longa is the one used as a flavor ingredient. The plant is originally from southern Asia and is widespread throughout India, Malaysia, Ceylon and Japan. It is a perennial herb whose rhizome yields (like that of ginger, which it also resembles) climbing stalks with leaves only or with leaves and flowers. Reproduction occurs through the splitting of the rhizome, which is the only part used (dried rhizome as is or after previously boiling in water). Turmeric has a spicy, fresh odor reminiscent of sweet orange and ginger and a slightly pungent, bitter flavor.
Chemical Properties
orange crystalline powder
Physical properties
Appearance: orange-brown crystalline powder and tastes a little bitter. It will turn into reddish brown in alkaline solution and yellow in neutral and acidic solution. It has strong stability against the reducing agent. It has excellent pigmentation which is not easy to fade. It is sensitive to light, heat, and iron ion. When PH is greater than
8, curcumin turns from yellow to red, which can be used as a pH indicator.
Solubility: insoluble in water or diethyl ether and soluble in ethanol, propylene
glycol, acetic acid, and alkali solution.
Melting point: about 183 °C.
History
Curcumin is one such agent that was described about two centuries ago as the yellow coloring matter from the rhizomes of Curcuma longa. Besides curcumin, more than 300 different components, including phenolics and terpenoids, have been identified in turmeric, but curcumin is one of the most important active components . Pure curcumin was prepared in 1842 by Vogel Jr. After 1870, the possible structure of curcumin was reported by several chemists in the subsequent decades. The chemical structure of curcumin as diferuloylmethane or 1,6-heptadiene-3,5-dione-1,7-bis (4-hydroxy-3-methoxyphenyl)-(1E, 6E) was reported by Milobedzka et?al. (1910). Lampe and Milobedzka (1913) reported the synthesis of curcumin. However, Srinivasan (1953) for the first time used chromatography to separate and quantify the components of curcumin .
Jiang Huang has been used for more than 6000 years; it is also well known for its
medicinal value and active ingredients. But it was not until the middle of the twentieth century that scientists conducted a systematic study on their pharmacological
effects. In 1949, Schraufstatter and Bernt found that curcumin has a variety of antibacterial effects against Streptococcus, Salmonella, Mucor, Mycobacterium and so
on . In the 1970s, the study also found that it has lipid-lowering, anti-inflammatory, antioxidant, and antidiabetic effects. In 1980s, it was found to have antitumor
effects. In the last 30 years, there are many reports about the clinical and pharmacological effects of curcumin.
At present, more than 65 human clinical trials have been completed, and more
than 35 clinical trials are in progress. In addition, the study of curcumin derivatives
has also become a hot topic in recent years.
Uses
antiedemic, antiinflammatory, bile stimulant; antibacterial, antifungal, lipo/cyclooxygenase inhibitor
Uses
A natural phenolic compound. Potent anti-tumor agent having anti-inflammatory and anti-oxidant properties. Induces apoptosis in cancer cells and inhibits phorbol ester-induced protein kinase C (PKC) activity. Reported to inhibit production of inflammatory cytokines by peripheral blood monocytes and alveolar macrophages. Potent inhibitor of EGFR tyrosine kinase and IκB kinase. Inhibits inducible nitric oxide synthase (iNOS), cycloxygenase and lipoxygenase. Easily penetrates into the cytoplasm of cells, accumulating in membranous structures such as plasma membrane, endoplasmic reticulum and nuclear envelope.
Uses
Curcumin is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family (Zingiberaceae). The curcuminoids are polyphenols and are responsible for the yellow color of turmeric. Curcumin can exist in at least two t
Uses
For preparing curcuma paper, pH range 8-9. In the detection of boron.
Definition
ChEBI: A beta-diketone that is methane in which two of the hydrogens are substituted by feruloyl groups. A natural dyestuff found in the root of Curcuma longa.
General Description
Orange-yellow needles.
Air & Water Reactions
Slightly soluble in hot water .
Reactivity Profile
Curcumin is sensitive to light and changes in pH. Curcumin may react with oxidizing materials.
Biological Activity
Antitumor, anti-inflammatory and antioxidant agent. Downregulates expression of reactive-oxygen-generating enzymes (cyclooxygenase, lipoxygenase, iNOS), TNF α , IL-1, IL-6, PKC, EGFR, NF- κ B, I κ B kinase and more. Upregulates expression of PPAR γ , p53, Nrf2. Also displays antimicrobial, antidiabetic neuro- and cardioprotective properties in vivo .
Biochem/physiol Actions
A natural phenolic compound. Potent anti-tumor agent having anti-inflammatory and anti-oxidant properties. Curcumin has been cited as a potential chemopreventive agent, in addition to its chemotherapeutic activity. Induces apoptosis in cancer cells and inhibits phorbol ester-induced protein kinase C (PKC) activity. Reported to inhibit production of inflammatory cytokines by peripheral blood monocytes and alveolar macrophages. Potent inhibitor of EGFR tyrosine kinase and IκB kinase. Inhibits inducible nitric oxide synthase (iNOS), cycloxygenase and lipoxygenase. Easily penetrates into the cytoplasm of cells, accumulating in membranous structures such as plasma membrane, endoplasmic reticulum and nuclear envelope.
Mechanism of action
Curcumin, the active component of turmeric (Curcuma longa), has been regarded as an anti-inflammatory and antioxidant agent . Particularly, it can scavenge reactive oxygen species, such as hydroxyl radicals, superoxide anion radicals, and nitrogen dioxide radicals. Additionally, it serves as an anti-inflammatory by down-regulating the production of pro-inflammatory cytokines (e.g., IL-1 and TNF-α) and inhibiting the activation of specific transcription factors (e.g., NF-κB and AP-1). Curcumin also demonstrates antiproliferative properties. Specifically, it inhibits UV radiation-induced skin cancer in SKH-1 hairless mice and reduces UVB-induced matrix metalloproteinase-1/3 expression in human dermal fibroblasts via MAPK-p38/JNK pathway suppression.
Pharmacology
1. Anti-fibrosis effects: curcumin has the effect of anti-fibrosis in the lung, liver, kidney, and so on. It could inhibit the release of various inflammatory factors and reduce the expression of collagen, laminin, hyaluronic acid, and other extracellular matrix content. It could also reduce the transforming growth factors such as TGF-尾 to inhibit cell proliferation .
2. Antitumor effects: the antitumor effect of curcumin is currently the most studied
pharmacological effects and attracts a lot of attention worldwide. Curcumin has
been proved to inhibit the proliferation of a variety of tumor cells through regulating a variety of transcription factors (NF-κB, AP-1, etc.), mitogen-activated
protein kinase (MAPK), growth factor receptor kinase (PDGFR, VEGFR, etc.),
and cyclooxygenase. It plays an important role in the cell cycle and further to
inhibit proliferation. Curcumin can also inhibit the migration of tumor cells by
activating caspase and inducing tumor cell apoptosis .
3. Anti-inflammatory effects: curcumin has a strong inhibitory effect on different
kinds of inflammation. The mechanism might relate to the reduction of the
expression of prostaglandins and leukotriene to decrease the release of various
inflammatory factors. The anti-inflammatory effect of curcumin is close to that
of nonsteroidal anti-inflammatory drugs and glucocorticoids, but it has higher
safety and lower side effects .
4. Antimicrobial effects: curcumin has a strong inhibitory effect on bacteria,
viruses, fungi, and parasites . Researchers believe that curcumin may play a
role in inhibiting microbial survival and reproduction by destroying microbial
cell membranes, inducing their genetic changes, and so on.
5. Hypolipidemic effect: many researchers believe that curcumin will become a
hypolipidemic drug with a good prospect. It can lower the levels of total blood
cholesterol and triglyceride levels, increase apolipoprotein A level, promote lowdensity lipoprotein (LDL) metabolism, and increase LDL excretion to reduce
LDL body content .
6. Drug metabolism: rats were treated with a single dose of refined curcumin orally,
60–65% of which was absorbed by the gastrointestinal tract. Within 5 days, 40%
of curcumin were excreted from the feces. The plasma concentration reached the
peak after 3 days. The transformation of curcumin happened in the process of
hepato-enteral circulation .
Anticancer Research
It is a yellow-colored polyphenolic compound found in turmeric and used as a foodadditive. It has antitumor effects involved in mutagenesis, cell cycle regulation,apoptosis, oncogene expression, and metastasis. Thus it regulates the initiation,promotion, and progression of disease (Hosseini and Ghorbani 2015). Its mechanismof action is diversified and convoluted. 10 μM curcumin suppresses binding of theTPA response element (TRE) by c-Jun/activator protein-1 in NIH 3 T3 cells ofmouse fibroblasts. Both protein kinase C and ornithine decarboxylase are alsoinhibited by curcumin. Inhibition of cyclooxygenase and lipoxygenase leads tosuppression of arachidonic acid cascade (Murakami et al. 1996). Curcumin is animpressive blocker of the activation of NF-κB by inhibiting IκB kinase (IKK).Curcumin also downregulates cyclin D1, suppresses the cell growth, and inducesapoptosis in prostate, breast, acute myelogenous leukemia, and multiple myelomacancer cells. It may act against psoriasis by inhibition of phosphorylase kinaseenzyme (Aggarwal and Shishodia 2004). Curcumin downregulates the TNF-inducedNF-κB-regulated gene products involved in cellular proliferation (cyclin D1, COX-2,c-myc), antiapoptosis (IAP2, IAP1, Bcl-2, XIAP, Bcl-xL, TRAF1, Bf1–1/A1,Cflip), and metastasis (MMP-9, VEGF, ICAM-1). It also suppresses the activity ofIκBα kinase, κBα degradation, IκBα phosphorylation, p65 nuclear translocation,p65 phosphorylation, and p65 acetylation (Aggarwal et al. 2008). It upregulates the expression of p53, p16, p21, EGR1 (early growth response protein1), ERK(extracellular signal-regulated kinase), JNK(c-Jun-N-terminal kinase), ElK1, Bax,and caspase 3, caspase8, and caspase9 proteins and downregulates Bcl2, mTOR,p65, Bcl-xL, AKT, EGFR, cdc2, retinoblastoma protein (Prb), c-myc, and cyclin D1proteins (Singh et al. 2016b). It can dissociate raptor from mTOR and inhibit mTORcomplex1. The inhibition of the Akt/mTOR signaling results from thedephosphorylation dependent on the calyculin A-sensitive protein phosphatase.Further, it modulating effect on AP-1 in HT-29 human colon cancer cells was foundto be a dose-dependent increase of AP-1 luciferase activity (Ravindran et al. 2009).
Curcumin is a dynamic element of turmeric, an outstanding Indian zest that isobtained from the plant Curcuma longa dried roots. Curcumin hindered PDGFR-incitedproliferation of human hepatic myofibroblasts (Zheng and Chen 2006). Theactivated mechanism by curcumin in PDGF signaling is as follows: Curcumindecreases the level of tyrosine phosphorylation of PDGFR-β and EGF-R; repressesthe action of ERK, JNK, and PI3/AKT; reduces cell growth; and induces apoptosisdose-dependently (Kunnumakkara et al. 2008). Moreover, curcumin interferes withPDGF signaling via relieving its inhibitory effect on PPARγ gene expression toreduce the cell growth; it also promotes the expression of PPARγ genes (Zhou et al.2007).
This compound is a yellow pigment produced by plants, mostly by those in theginger family (Zingiberaceae). Curcumin has enormous potential in terms of cancerprevention and treatment, and numerous studies and reviews described it as a potentantioxidant and anti-inflammatory agent (Aggarwal et al. 2003; Agrawal and Mishra2010). It inhibits biochemical activity, restraining overexpression of some signallingpathways and regulating the expression of tumour suppression genes (Cre?uet al. 2012). Temu kunci, or galangal (Boesenbergia pandurata), is a rhizome generallyused in cooking that can also be prepared to treat diarrhoea and mouth ulcers.It has been proven non-toxic to human skin fibroblast cells and offers protectiveeffects against colon cancer (Kirana et al. 2007). Turmeric (Curcuma longa) andginger (Zingiber officinale) are two plants that contain an abundance of curcuminand which have been investigated for their therapeutic properties. One piece ofresearch, for example, showed that ethanolic extract of turmeric showed anti-melanomaactivity against malignant melanomas (Danciu et al. 2015).
Clinical Use
1. Cholagogic effect could promote bile formation and secretion.
2. Hypolipidemic effect could reduce the level of cholesterol in the blood and prevent atherosclerosis.
3. Antibacterial and antiviral effect could inhibit Staphylococcus aureus and HIV.
4. Liver protection.
5. Anticancer and antitumor effect.
6. Help with the prevention of dementia.
7. Anti-inflammation and treatment of acne and dermatitis.
8. There are no reports of adverse effect of curcumin till now.
Purification Methods
Crystallise curcumin from EtOH or acetic acid. [Beilstein 8 IV 3697.]
References
1) Zhang?et al.?(2015),?Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse; Cell Immunol.?298?88 2) Li?et al.?(2018),?Anticancer effects of curcumin on nude mice bearing lung cancer A549 cell subsets SP and NSP cells; Oncol. Lett.?16?6756
Curcumin Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 | sales002@sdzschem.com | China | 3050 | 58 |
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 | sales06@tgybio.com | China | 961 | 58 |
| Xi’an Sengmei Biotech Co.,Ltd. | +8615398038360 | lionel@accenturebio.com | China | 288 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| Chengdu ChenLv Herb Co.,Ltd | +undefined13608205856 | maryextract@126.com | China | 127 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 359 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 | Jany1001@kangcang.com.cn | China | 338 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 864 | 58 |
Related articles
- The role of curcumin in food additives and preservatives
- Curcumin is a natural compound with good anti-inflammatory and anticancer properties.
- Apr 11,2022
- Curcumin as an Agent for Rapid Healing of Burns, and UV Light Injury and Photodamaged Skin
- The ability of topical curcumin to assist in skin repair after injury may be a very important effect that accounts for its wid....
- Mar 3,2022
- Curcumin in Cosmetics: Biochemical Basis for Skin Repair with Use of Topical Curcumin
- Curcumin is the active ingredient in turmeric, derived from the rhizome of Curcuma longa. Turmeric has been used in cosmetics ....
- Mar 3,2022
View Lastest Price from Curcumin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Curcumin
458-37-7
|
US $9.00-70.00 / g | 10g | 99% | 10 tons | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-24 | Ferric chloride hexahydrate
458-37-7
|
US $9.00-70.00 / g | 10g | 99% | 10 tons | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-23 | Curcumin
458-37-7
|
US $200.00-85.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd |
-

- Curcumin
458-37-7
- US $9.00-70.00 / g
- 99%
- Hebei Kangcang new material Technology Co., LTD
-

- Ferric chloride hexahydrate
458-37-7
- US $9.00-70.00 / g
- 99%
- Hebei Kangcang new material Technology Co., LTD
-

- Curcumin
458-37-7
- US $200.00-85.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd