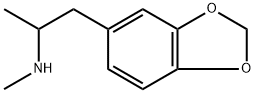
(+/-)-3,4-METHYLENEDIOXYMETHAMPHETAMINE
- CAS No.
- 42542-10-9
- Chemical Name:
- (+/-)-3,4-METHYLENEDIOXYMETHAMPHETAMINE
- Synonyms
- Methylenedioxymethamphetamine;XTC;3,4-MDMA;C07577;ecstacy;DL-MDMA;(+/-)-MDMA;AURORA KA-7748;(±)-MDMA solution;3,4-METHYLENDIOXYMETHAMPHETAMIN
- CBNumber:
- CB4221584
- Molecular Formula:
- C11H15NO2
- Molecular Weight:
- 193.25
- MDL Number:
- MFCD00287291
- MOL File:
- 42542-10-9.mol
| Boiling point | bp0.4 100-110° |
|---|---|
| Density | 1.100±0.06 g/cm3(Predicted) |
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| pka | 10.32±0.10(Predicted) |
| InChIKey | SHXWCVYOXRDMCX-UHFFFAOYSA-N |
| EWG's Food Scores | 1 |
| FDA UNII | KE1SEN21RM |
| NCI Drug Dictionary | 3,4-methylenedioxymethamphetamine |
| EPA Substance Registry System | 1,3-Benzodioxole-5-ethanamine, N,.alpha.-dimethyl- (42542-10-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H225-H301+H311+H331-H370 |
| Precautionary statements | P210-P260-P280-P301+P310-P311 |
| Hazard Codes | F,T |
| Risk Statements | 11-23/24/25-39/23/24/25 |
| Safety Statements | 7-16-36/37-45 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 1 |
| Toxicity | A novel psychoactive drug chemically related to the hallucinogenic agent MDA, but reported to be non-hallucinogenic. Obtained by direct synthesis, at high doses it is reported to show a stimulant-like effect in animals. Its LD50 is 97 mg/kg, i. p., 49 mg/kg, i.p., 14 mg/kg, i.v., and 22 mg/kg, i.v. in mice, rats, dogs, and monkeys, respectively. At higher doses, MDMA has pressor effects and produces tachycardia, and causes damage to serotonin neurons in both rats and monkeys. The (S)- (1)-enantiomer is more active than the (R)-(?′)-enantiomer and, like amphetamine, the pharmacological effects may be due to a release of endogenous monoamine neurotransmitter, probably serotonin and/or norepinephrine. MDMA is also an inhibitor of monoamine uptake into brain synaptosomes. Symptoms include pronounced mydriasis, with nystagmus and jaw clenching, and nausea also is often reported. This substance produces a feeling of well-being and euphoria and is similar in some respects to MDA, in that it seems to enhance a sense of empathy and emotional openness in users. Toxic reactions would appear to occur as a result of the pressor action, and sympathomimetic effects of the drug. No appropriate therapeutic intervention has been reported. Peripheral adrenergic blocking agents, or chlorpromazine, have been used, however, to prevent death in dogs following a lethal i.v. dose of the chemically related agent MDA. |
(+/-)-3,4-METHYLENEDIOXYMETHAMPHETAMINE Chemical Properties,Uses,Production
Uses
3,4-Methylenedioxymethamphetamine (MDMA) is an Entactogen; It is the N-methyl derivative of MDA. The (S)-form of MDMA possesses more potent CNS activity than the (R) form of MDMA.MDMA is controlled substance (hallucinogen).
Definition
ChEBI: A member of the class of benzodioxoles that is 1,3-benzodioxole substituted by a 2-(methylamino)propyl group at position 5.
Metabolic pathway
Four biotransformation pathways of 3,4- (methylenedioxy)methamphetamine (MDMA) in rats are identified as N-demethylation, O-dealkylation, deamination, and conjugation (O-methylation, O- glucuronidation, and/or sulfation). Specific MDMA metabolites identified are 3-hydroxy-4- methoxymethamphetamine, 4-hydroxy-3- methoxymethamphetamine, 3,4- dihydroxymethamphetamine, 4-hydroxy-3- methoxyamphetamine, 3,4- (methylenedioxy)amphetamine (MDA), (4-hydroxy-3- methoxyphenyl)acetone, [3,4- (methylenedioxy)phenyl]acetone, and (3,4- dihydroxyphenyl)acetone. MDMA is metabolized by 10 000 g rat liver supernatant to 4-hydroxy-3- methoxymethamphetamine, 3,4- dihydroxymethamphetamine, MDA, and [3,4- (methylenedioxy)phenyl]acetone. Also, the 10 000 g rat brain supernatant metabolizes MDMA to 4-hydroxy- 3-methoxymethamphetamine, 3,4- dihydroxymethamphetamine, 4-hydroxy-3- methoxyamphetamine, and MDA.