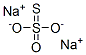Sodium thiosulfate
- CAS No.
- 7772-98-7
- Chemical Name:
- Sodium thiosulfate
- Synonyms
- SODIUM THIOSULPHATE;Thiosulfate;SODIUM HYPOSULFITE;HYPO;Sodium Thiosulfite;Sodium Thiosulfat;Anhydrous sodium thiosulfate;Sodium Hyposulfite Anhydrous;'HYPO';Detoxol
- CBNumber:
- CB4232650
- Molecular Formula:
- Na2O3S2
- Molecular Weight:
- 158.11
- MDL Number:
- MFCD00003499
- MOL File:
- 7772-98-7.mol
- MSDS File:
- SDS
| Melting point | 48°C |
|---|---|
| Boiling point | 100°C |
| Density | 1.01 g/mL at 25 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Immiscible with alcohol. |
| form | Solid |
| Specific Gravity | 1.667 |
| color | White |
| PH | 6.0-8.5 (25℃, 50mg/mL in H2O) |
| Water Solubility | Soluble in water. Insoluble in alcohol. |
| Sensitive | Hygroscopic |
| Merck | 14,8694 |
| Stability | Stable. Incompatible with strong acids, strong oxidizing agents, iodine, mercury. |
| InChIKey | AKHNMLFCWUSKQB-UHFFFAOYSA-L |
| LogP | -0.428 (est) |
| CAS DataBase Reference | 7772-98-7(CAS DataBase Reference) |
| FDA 21 CFR | 184.1807; 582.6807; 310.545 |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | sodium thiosulfate |
| FDA UNII | L0IYT1O31N |
| NCI Drug Dictionary | sodium thiosulfate |
| ATC code | V03AB06 |
| EPA Substance Registry System | Sodium thiosulfate (7772-98-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H335-H315-H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 24/25-23-36-26 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | XN6476000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28323000 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg | |||||||||
| NFPA 704 |
|
Sodium thiosulfate price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 909173 | Sodium thiosulfate free-flowing, Redi-Dri?, ReagentPlus?, 99% | 7772-98-7 | 250g | $73.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 909173 | Sodium thiosulfate free-flowing, Redi-Dri?, ReagentPlus?, 99% | 7772-98-7 | 500g | $101 | 2024-03-01 | Buy |
| Sigma-Aldrich | 909173 | Sodium thiosulfate free-flowing, Redi-Dri?, ReagentPlus?, 99% | 7772-98-7 | 1kg | $169 | 2024-03-01 | Buy |
| Sigma-Aldrich | 217263 | Sodium thiosulfate ReagentPlus , 99% | 7772-98-7 | 1kg | $160 | 2024-03-01 | Buy |
| Sigma-Aldrich | 217263 | Sodium thiosulfate ReagentPlus , 99% | 7772-98-7 | 2.5kg | $275 | 2024-03-01 | Buy |
Sodium thiosulfate Chemical Properties,Uses,Production
Description
Sodium thiosulfate is a commonly used chemical material, as the fixing agent in photography, film and printing plate-making industry, as a reducing agent used in tanning. It is used as a bleaching agent for removing residual and a mordant in the paper and textile industries, as antidotes of cyanide poisoning in medicine, as dechlorination agent and fungicides of drinking water and wastewater in water treatment, as a copper corrosion inhibitor of circulating cooling water and a deoxidizer of boiler water systems. It is also used for Cyanide wastewater treatment. Soda ash and sulfur are generally used as raw materials in industry, soda ash reacts with sulfur dioxide produced by the combustion of sulfur to produce sodium sulfite, then add sulfur for boiling reactions, and then filter, bleach, concentrate and crystallize, etc, can obtain sodium thiosulfate pentahydrate. Other production waste containing sodium sulfide, sodium sulfite, sulfur and sodium hydroxide can also be used, after appropriate treatment to get the product.

The above information is edited by Yan Yanyong of Chemicalbook.
Physical properties
Sodium thiosulfate has two forms as anhydrous sodium thiosulfate (Na2S2O3) and sodium thiosulfate pentahydrate (Na2S2O3 · 5H2O), the latter is also known as soda and Hyperion. A molecular weight is 158.11 and 248.18 respectively. Anhydrous sodium thiosulfate is an opaque crystalline powder. The relative density is 1.667. It is odorless, salty taste, soluble in water, insoluble in ethanol. It decomposes in acidic solution, has a strong reduction, easily absorbs moisture. Sodium thiosulfate pentahydrate is odorless, colorless and transparent monoclinic crystals or granules. The relative density is 1.685. The melting point is 48 ℃. It loses all crystal water at 100 ℃, and decomposes above 100 ℃. It is easily weathered in dry and hot air, slightly deliquescence in the hot and humid air. It is soluble in water and turpentine, insoluble in ethanol, decomposes in case of strong acid. Prolonged exposure in the air, it is easily oxidated and carbonized by oxygen and carbon dioxide.
Chemical Properties
It is colorless monoclinic crystal or white crystalline powder, Odorless, salty ignorant. The relative density is 1.667. Soluble in water, the solubility is 231 g/100 ml of water at 100 ℃. Insoluble in alcohol. deliquescence easily in air. With a strong reductibility, decomposition in acidic solution.
Reactions
With reductibility, sodium thiosulfate is a medium strength reducing agent, for example, can be oxidated by chlorine:
Na2S2O3 + 4Cl2 + 5H2O = Na2SO4 + H2SO4 + 8HCl
this reaction is used in textile and paper industries, and sodium thiosulfate is used as dechlorination agent. Again, it can be oxidized to sodium tetrathionate by iodine:
Na2S2O3 + I2 = Na2S4O6 + 2Nal
this reaction is used in analytical chemistry for quantitative determination of iodine.
It is also used in tanning, extracts silver from the ore and so on. Since there are different oxidation states of sulfur in sodium thiosulfate molecule, it is stable in alkaline medium, unstable an acidic medium, oxidation-reduction reaction occurs when acidification, separate out sulfur:
Na2S2O3 + H2SO4 = Na2SO4 + H2S2O3 H2S2O3 = H2SO3 + S H2SO3 = H2O + S02.
With coordination, thiosulfate ions can form stable complexes with certain metal ions, for example, silver bromide can be dissolved in an aqueous solution of hypo to produce stable [Ag (S2O3) 2] 3-complex ions:
AgBr + 2Na2S2O3 = Na3 [Ag (S2O3) 2] + NaBr, so it is commonly used as a photographic fixing bath. Sodium sulfite and sulfur are boiled, and then filtration, evaporation, crystallization to prepare sodium thiosulfate.
Uses
1. Sodium thiosulfate is Used in manufacture of the fixing powder and preparation of the photographic industry fixer, desiccant. Used as a reducing agent of dichromate in leather tanning industry.
2. Sodium thiosulfate is an important inorganic chemical raw material. It can be used in the synthesis of pesticides Bisultap, Thiosultap disodium, CARTAP and so on.
3. Used as analytical reagents, mordant and fixer.
4. Used as new insecticides, with stomach poisoning, uptake and contact action effects, it can prevent and treat rice yellow stem borer, stem borer, leaf roller, rice thrips and vegetables, fruit pests.
5. With uptake and strong contact, stomach poisoning effects, it can prevent and treat various rice pests and vegetables, fruit pests.
6. Used as the chlorine removal agent for Pulp and cotton fabric bleached, as chelating agents, anti-oxidants in the food industry, as detergents, disinfectants in the pharmaceutical industry.
7. Used as chelating agents, antioxidants, anti-browning agent, as the dechlorination agent of chlortetracycline hydrochloride (or chlortetracycline) chilled with ice, dechlorination reaction is as follows:
More chlorine content will cause side reactions of free sulfur, so only used for very dilute concentrations of chlorine.
Used as salt chelating agent, limited content is 0.1% or less.
Preparation
Sodium thiosulphate is an important chemical commodity that is known to photographers as "hypo". It can be prepared by the reaction of sodium sulphite and the bisulphite with H2S:
2Na2S03 + 2NaHS03 + 2H2S -> 3Na2S203 + 3H20
It can also be prepared by the reaction of sulphur with sodium sulphite above 60 °C
Na2SO3 + S -> Na2S2O3 and by the reaction of H2S and permanganate.
Sodium thiosulphate decomposes at 310°C to form sulphur and Na2S03 , and at 400°C to form Na2S4 and Na2S. It absorbs S02 to form free sulphur and Na2S04 . Sodium thiosulphate forms many hydrates and their solubility relationships are quite complex.
Production method
1. It is generated by heat of a solution of sodium sulfite and sulfur powder.
2. There are more synthesis methods of sodium thiosulfate, such as sodium sulfite method, sodium sulfide method and so on.
Sodium sulfite method
Soda solution reacts with sulfur dioxide gas, and caustic soda was added, adding sodium sulfide to remove impurities by filtration, and then sulfur powder was dissolved in a hot solution of sodium sulfite to react, filter, remove impurities, and then filter repeatedly, add caustic alkali to process, through concentration, filtration, crystallization, centrifugal dewatering, screening, to obtain the finished product of sodium thiosulfate. Reaction equation: Na2CO3 + SO2 = Na2SO3 + CO2 ↑
Na2SO3 + S + 5H2O = Na2S2O3 · 5H20
Sodium sulfide method
A raw material liquid formulated from sodium sulfide evaporation residue, barium sulfide wastewater (containing sodium carbonate and sodium sulfide) reacts with sulfur dioxide, sulfur powder was added after clarification, heated to react, evaporation, cooling crystallization, washing, separation, screening to obtain sulfur thiosulfate products. Reaction equation: 2Na2S + Na2CO3 + 4SO2 = 3Na2S2O3 + CO2 ↑
2Na2S + 3Na2CO3 + 6SO2 + 2S = 5Na2S2O3 + 3CO2 ↑
3. Dehydration method is that heat sodium thiosulfate pentahydrate crystalline indirect with steam, it dissolves into itself crystal water, concentration, centrifugal dewatering, drying, screening, to obtain the finished product of anhydrous sodium thiosulfate.
Na2S2O3 · 5H20 → Na2S2O3 + 5H2O
Chemical Properties
Sodium thiosulfate, Na2S203·5H20, also known as sodium hyposulfite, hypo,andsodium subsulfite, is a white crystalline solid that has a melting point of 48 °C(118 °F). Water soluble,it is used as a fixing agent for photographic films, plates, and papers. Sodium thiosulfate is used in medicine, as a germicide, in manufacturing leather, as a mordant in dyeing, and for extracting silver from ore.
Physical properties
Anhydrous thiosulfate is a white powder; soluble in water; insoluble in ethanol.
Sodium pentahydrate is a colorless, odorless, crystalline solid; density 1.69 g/cm3; decomposes around 50°C; effloresces in dry air above 33°C; very soluble in water and oil of turpentine; insoluble in ethanol.
Uses
Sodium thiosulfate (thiosulfate) Known simply as “hypo,” an abbreviated form of the incorrect form hyposulfite, this white crystal was made by boiling calcium thiosulfate with sodium sulfate. It is soluble in water and oil of turpentine but not in alcohol. The property of sodium thiosulfate to dissolve silver halides was discovered in 1819 by Sir John Herschel and was probably first used to fix photographic images by L. J. M. Daguerre. After Daguerre published its use in his 1839 manual W. H. F. Talbot finally used it to fix calotype negatives and silver chloride prints, although he continued to stabilize them with potassium iodide and sodium chloride presumably for the variety of colors possible. Sodium thiosulfate was the primary fixing agent throughout the 19th century and is still used today.
Uses
Sodium Thiosulfate is a sequestrant, antioxidant, and formulation aid that is a powder soluble in water. it can be used in alcoholic bev- erages at 5 ppm and in table salt at 0.1%. it is also termed sodium hyposulfite.
Uses
Sodium thiosulfate is a common analytical reagent used in iodometric titration to analyze chlorine, bromine, and sulfide. Other uses are in bleaching paper pulp, bleaching straw, ivory, and bones, for removing chlorine from solutions, silver extraction from its ores, a mordant in dyeing and printing textiles, and as an antidote to cyanide poisoning.
Another major application is in photography, where it is used as a fixer to dissolve unchanged silver salts from exposed negatives.
Sodium Thiosulfate is used primarily as a medicament against cyanide poisoning, able to convert cyanide into thiocyanate, a reaction which is catalyzed by the enzyme Rhodanese. Antioxidant.
Definition
ChEBI: An inorganic sodium salt composed of sodium and thiosulfate ions in a 2:1 ratio.
Preparation
Sodium thiosulfate is a common reducing agent. It reduces iodine to iodide anion forming sodium tetrathionate. This reaction is utilized in the so-called iodometric titration: 2S2O32ˉ + I2 → S4O62ˉ + 2Iˉ
Sodium thiosulfate reacts with chlorine to form sodium bisulfate and hydrochloric acid. This reaction removes chlorine from aqueous solutions:Na2S2O3 + 4Cl2 + 5H2O → 2NaHSO4 + 8HCl
Sodium thiosulfate reacts with hydrochloric acid, decomposing to sulfur and sulfur dioxide: Na2S2O3 + 2HCl → 2NaCl + S + SO2 + H2O.
brand name
Sulfactol (Sterling Winthrop).
General Description
Sodium thiosulfate (Na2S2O3) can be obtained from Na2S2O3.10H2O by heating above 100 °C. It can be synthesized from sodium sulfate.
Hazard
Use in foods restricted to 0.1%.
Safety Profile
Moderately toxic by subcutaneous route. Incompatible with metal nitrates, sodium nitrite. When heated to decomposition it emits very toxic fumes of Na2O and SOx. See also SODIUM THIOSULFATE and SODIUM THIOSULFATE, PENTAHYDRATE.
Veterinary Drugs and Treatments
Sodium thiosulfate (alone or in combination with sodium nitrite)
is useful in the treatment of cyanide toxicity. It has been touted for
use in treating arsenic or other heavy metal poisonings, but its efficacy
is in question for these purposes. However, because sodium
thiosulfate is relatively non-toxic and inexpensive, it may be tried
to treat arsenic poisoning. When used in combination with sodium
molybdate, sodium thiosulfate may be useful for the treatment of
copper poisoning.
Sodium thiosulfate may be useful for the topical treatment for
some fungal infections (Tinea). In humans, sodium thiosulfate has
been used to reduce the nephrotoxicity of cisplatin therapy. A 3 or
4% solution has been used to infiltrate the site of extravasations of
cisplatin, carboplatin, or dactinomycin. In combination with steroids,
sodium thiosulfate may reduce the healing time associated
with doxorubicin extravasation.
Purification Methods
Crystallise it from EtOH/H2O solutions or from water (0.3mL/g) below 60o by cooling to 0o, and dry it at 35o over P2O5 under vacuum. [Foerster & Mommsen Chem Ber 57 258 1924.] This salt is used as a secondary standard in volumetric analysis [Kilpatrick J Am Chem Soc 45 2132 1923], and is used as “Hypo” in photography [Hargreaves & Dunningham J Soc Chem Ind 42 147T 1923.]
Sodium thiosulfate Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 874 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 1696 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
Related articles
- Medical uses of Sodium thiosulfate
- Sodium thiosulfate (STS) is an industrial chemical which also has a long medical history. It was originally used as an intrave....
- Nov 14,2019
View Lastest Price from Sodium thiosulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-25 | Sodium thiosulfate
7772-98-7
|
US $0.00 / Kg/Bag | 1KG | 99% | 500mt/month | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-24 | Sodium thiosulfate
7772-98-7
|
US $320.00 / ton | 5ton | 99% | 3000tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-24 | Sodium thiosulfate
7772-98-7
|
US $370.00-310.00 / ton | 1ton | 99% | 80ton | Hebei Dangtong Import and export Co LTD |
-

- Sodium thiosulfate
7772-98-7
- US $0.00 / Kg/Bag
- 99%
- Jinan Finer Chemical Co., Ltd
-

- Sodium thiosulfate
7772-98-7
- US $320.00 / ton
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Sodium thiosulfate
7772-98-7
- US $370.00-310.00 / ton
- 99%
- Hebei Dangtong Import and export Co LTD