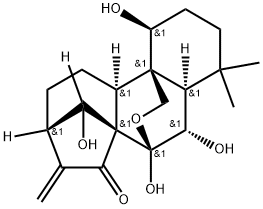
Oridonin
- CAS No.
- 28957-04-2
- Chemical Name:
- Oridonin
- Synonyms
- ,14r)-;Rubesin;ORIDONIN;ISODONAL;Isodonol;NSC 250682;Rubescenin;Oridonin A;Rubescensin;Oridonin,98%
- CBNumber:
- CB4236658
- Molecular Formula:
- C20H28O6
- Molecular Weight:
- 364.44
- MDL Number:
- MFCD00221762
- MOL File:
- 28957-04-2.mol
| Melting point | 248-250°C |
|---|---|
| Boiling point | 599.8±50.0 °C(Predicted) |
| Density | 1.42±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO: >20mg/mL |
| form | Yellow solid |
| pka | 10.96±0.70(Predicted) |
| color | White or off-white |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| InChIKey | SDHTXBWLVGWJFT-WNKSEERENA-N |
| SMILES | O[C@@]12OC[C@]3([C@H](CCC(C)(C)[C@@]3([H])[C@@H]1O)O)[C@]1([H])CC[C@@]3([H])C(=C)C(=O)[C@@]21C3([H])O |&1:1,4,5,11,13,16,20,26,r| |
| FDA UNII | 0APJ98UCLQ |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 40 |
| Safety Statements | 36/37-24/25 |
| WGK Germany | 2 |
| RTECS | NZ8177000 |
| HS Code | 29389090 |
Oridonin price More Price(56)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | O9639 | Oridonin ≥98% (HPLC) | 28957-04-2 | 5mg | $143 | 2024-03-01 | Buy |
| Sigma-Aldrich | 496915 | Oridonin | 28957-04-2 | 5mg | $172 | 2024-03-01 | Buy |
| Cayman Chemical | 25665 | Oridonin ≥98% | 28957-04-2 | 5mg | $47 | 2024-03-01 | Buy |
| Cayman Chemical | 25665 | Oridonin ≥98% | 28957-04-2 | 10mg | $78 | 2024-03-01 | Buy |
| Sigma-Aldrich | O9639 | Oridonin ≥98% (HPLC) | 28957-04-2 | 25mg | $562 | 2024-03-01 | Buy |
Oridonin Chemical Properties,Uses,Production
Description
Oridonin is a diterpenoid that has been found in R. rubescens and has anti-inflammatory and anticancer properties. It is an inhibitor of AKT1 and AKT2 (IC50s = 8.4 and 8.9 μM, respectively). Oridonin inhibits proliferation of KYSE70, KYSE410, and KYSE450 esophageal cancer cells in a dose-dependent manner, halts the cell cycle at the G2/M phase, and induces apoptosis when used at a concentration of 20 μM. It decreases the expression of cleaved poly(ADP-ribose) polymerase (PARP), caspase-3, caspase-7, and Bims and the protein levels of phosphorylated AKT and reduces AKT activity. Oridonin reduces tumor growth in patient-derived mouse tumor models when administered at doses of 40 and 160 mg/kg. Oridonin is also an inhibitor of NLRP3 inflammasome assembly and activation. It inhibits inflammation in wild-type, but not Nlrp3-/-, mice in a model of high-fat diet-induced type 2 diabetes when administered at a dose of 3 mg/kg.
Uses
Oridonin is an effective anticancer agent due to its ability to inhibit proliferation and induce apoptosis of human osteosarcoma cells. Blocks Wnt/β-catenin signalling.
Definition
ChEBI: Oridonin is an organic heteropentacyclic compound and ent-kaurane diterpenoid with formula C20H28O6 isolated from the leaves of the medicinal herb Rabdosia rubescens. It has a role as an antineoplastic agent, an angiogenesis inhibitor, an apoptosis inducer, an anti-asthmatic agent, a plant metabolite and an antibacterial agent. It is an organic heteropentacyclic compound, an enone, a cyclic hemiketal, a secondary alcohol and an ent-kaurane diterpenoid.
Mechanism of action
The anti-inflammatory effect of oridonin is primarily associated with suppressing the nuclear factor-kappa B (NF-κB) signaling pathway, reducing secretion of serum cytokines including interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and inhibiting expression and function of toll-like receptors 4 (TLR4) as well as the p38-mitogen activated protein kinase (p38-MAPK) in endometritis, diabetic nephropathy, vascular inflammation, acute lung injury, liver injury, inflammatory bowel disease, and sepsis. Oridonin was reported to inhibit NLRP3, a key component in the NLPR3 inflammasome, by targeting the Cys279 residue of NLRP3 in the NACHT domain[7].
Pharmacokinetics
The anti-inflammatory property of oridonin has been well documented in various immunological diseases. The compound is able to counteract the expression of COX-2 and NOS-2 in the murine RAW 264.7 macrophage cell line activated with LPS. The mechanism underlies the direct interference of the compound with the active region of NF-κB, thereby blocking its nuclear localisation and reducing inflammation (Leung et al., 2005). Shang et al. (2016) observed the efficacy of oridonin against RA-FLS proliferation. They found that oridonin inhibited cell proliferation and promoted cell apoptosis in IL-1β–treated FLS through phosphorylation of ERK1/2 and JNK in a dose-dependent manner. Oridonin is also reported to restrict the release of pro-inflammatory cytokines in LPS-activated RAW264.7 macrophages (Shang et al., 2016).
Side effects
Oridonin has shown prominent adverse effects, even toxicity, under specific circumstances in vitro and in vivo. It showed hepatotoxicity and hepatoprotective effects, which the pair of pharmacological activities seems to be a paradox. However, through the analysis, it is found that this is mainly related to the concentration of oridonin and the time of administration. Long-term administration and high-dose administration may cause liver damage. On the other hand, according to the chemical structure of oridonin, it may react covalently with the sulfhydryl group of some proteins, which can partly explain the reason for adverse reactions, even toxicity of oridonin in specific environments[6].
References
1) He et al. (2018), Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity; Nat. Commun., 9 2550
2) Huang et al. (2018), Oridonin inhibits vascular inflammation by blocking NF-kB and MAPK activation; Eur. J. Pharmacol., 826 133
3) Sun et al. (2018), Oridonin inhibits aberrant AKT activation in breast cancer; Oncotarget, 9 23878
4) Li et al. (2018), Oridonin inhibits migration, invasion, adhesion and TGF- 1-induced epithelial-mesenchymal transition of melanoma cells by inhibiting the activity of PI3K/Akt/GSK-3 signaling pathway; Oncol. Lett., 15 1362
5) Lu et al. (2018), Oridonin exerts anticancer effect on osteosarcoma by activating PPARγ and inhibiting Nrf2 pathway; Cell Death Dis., 9 15
6) Xiang Li. “Oridonin: A Review of Its Pharmacology, Pharmacokinetics and Toxicity.” Frontiers in Pharmacology (2021): 645824.
7) Xi Liu . “Oridonin and its derivatives for cancer treatment and overcoming therapeutic resistance.” Genes Diseases 8 4 (2021): Pages 448-462.
Oridonin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +8619164747840 | 13119157289@163.com | China | 2971 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3424 | 58 |
| Nanjing Dolon Biotechnology Co.,Ltd. | 18905173768 | sales@dolonchem.com | CHINA | 2972 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | sales@pioneerbiotech.com | China | 3000 | 58 |
View Lastest Price from Oridonin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Oridonin; Rabdosia rubescens extract
28957-04-2
|
US $0.00 / mg | 20mg | ≥98% HPLC | 1000kg | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-04-22 | Oridonin
28957-04-2
|
US $0.00 / g | 1g | HPLC≥98% | 100kg | Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | |
 |
2023-02-24 | Oridonin
28957-04-2
|
US $0.00 / mg | 5mg | ≥98%(HPLC) | 10 g | Shanghai Standard Technology Co., Ltd. |
-

- Oridonin; Rabdosia rubescens extract
28957-04-2
- US $0.00 / mg
- ≥98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- Oridonin
28957-04-2
- US $0.00 / g
- HPLC≥98%
- Shaanxi Cuikang Pharmaceutical Technology Co., Ltd
-

- Oridonin
28957-04-2
- US $0.00 / mg
- ≥98%(HPLC)
- Shanghai Standard Technology Co., Ltd.