Diglyme
- CAS No.
- 111-96-6
- Chemical Name:
- Diglyme
- Synonyms
- DIGLYME;2-METHOXYETHYL ETHER;DEDM;BIS(2-METHOXYETHYL) ETHER;1-Methoxy-2-(2-methoxyethoxy)ethane;DIMETHYLDIGLYCOL;iglyme;Di(2-Methoxyethyl) ether;glyme2;DIYGLME
- CBNumber:
- CB4302276
- Molecular Formula:
- C6H14O3
- Molecular Weight:
- 134.17
- MDL Number:
- MFCD00008503
- MOL File:
- 111-96-6.mol
- MSDS File:
- SDS
| Melting point | -64 °C (lit.) |
|---|---|
| Boiling point | 162 °C (lit.) |
| Density | 0.944 g/mL at 20 °C (lit.) 0.939 g/mL at 25 °C (lit.) |
| vapor density | 4.6 (vs air) |
| vapor pressure | 3 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 134.6 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Sparingly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | ≤10(APHA) |
| Odor | Mild ethereal. |
| Relative polarity | 0.244 |
| explosive limit | 1.4-17.4%(V) |
| Evaporation Rate | 0.36 |
| Viscosity | 1.14 mPa s 20 C |
| Water Solubility | Miscible |
| Sensitive | Hygroscopic |
| λmax |
λ: 225 nm Amax: 1.00 λ: 240 nm Amax: 0.50 λ: 260 nm Amax: 0.20 λ: 280 nm Amax: 0.08 λ: 320-400 nm Amax: 0.01 |
| Merck | 14,3165 |
| BRN | 1736101 |
| Dielectric constant | 7.2999999999999998 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. May be air or light sensitive. |
| InChIKey | SBZXBUIDTXKZTM-UHFFFAOYSA-N |
| LogP | -0.36 at 25℃ |
| CAS DataBase Reference | 111-96-6(CAS DataBase Reference) |
| EWG's Food Scores | 2-5 |
| FDA UNII | M4BH3X0MVZ |
| NIST Chemistry Reference | Ethane, 1,1'-oxybis[2-methoxy-(111-96-6) |
| EPA Substance Registry System | Diethylene glycol dimethyl ether (111-96-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H360FD | |||||||||
| Precautionary statements | P202-P210-P233-P240-P241-P308+P313 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 60-61-10-19 | |||||||||
| Safety Statements | 53-45 | |||||||||
| RIDADR | UN 3271 3/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | KN3339000 | |||||||||
| F | 3-10-23 | |||||||||
| Autoignition Temperature | 370 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29091990 | |||||||||
| Toxicity | LD50 orally in Rabbit: 4760 mg/kg | |||||||||
| NFPA 704 |
|
Diglyme price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.02934 | Diethylene glycol dimethyl ether (stabilised) for synthesis | 111-96-6 | 250ML | $47.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02934 | Diethylene glycol dimethyl ether (stabilised) for synthesis | 111-96-6 | 1L | $160 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02934 | Diethylene glycol dimethyl ether (stabilised) for synthesis | 111-96-6 | 2.5L | $352 | 2024-03-01 | Buy |
| Sigma-Aldrich | M14102 | Diethylene glycol dimethyl ether ReagentPlus?, 99% | 111-96-6 | 20L | $1290 | 2023-06-20 | Buy |
| Sigma-Aldrich | 04143 | Diethylene glycol dimethyl ether analytical standard | 111-96-6 | 1mL | $28.5 | 2022-05-15 | Buy |
Diglyme Chemical Properties,Uses,Production
Description
Bis (2-methoxyethyl) ether, also known as diglyme, is a linear aliphatic diether widely used as a solvent and present as a clear liquid at room temperature with a mild ether odor. The compound is notknown to occur in nature. It is synthesized from ethylene oxide and methanol in the presence of either acidic or basic catalysts. The reaction is based on the classic Williamson ether synthesis. It can also be produced from diethylene glycol and dimethyl sulfate. In June 2012, ECHA proposed addition of diglyme to the REACH very high concern list.
Chemical Properties
Diethylene glycol dimethyl ether is a clear, water-white neutral liquid of faint, pleasant odor. This ether may be used as a solvent for alkali metal hydrides for use in such reactions as reduction, alkylation and condensation. It may also be used as a lacquer solvent.
Uses
Bis (2-methoxyethyl) ether, due to being chemically inert and possessing excellent solvent properties, is mainly used as a solvent and an anhydrous reaction medium for organometallic synthesis. It is also used as a solubilizer.
Uses
Solvent; it is used as reaction medium for Grignard and similar synthesis.
Uses
Diethylene glycol dimethyl ether is used as a solvent in organic reactions due to its stability towards higher pH and its high boiling point. It is particularly involved in reactions utilizing organometallic reagents such as Grignard reactions and metal hydride reductions. It is also a solvent for hydroboration reactions with diborane.
Definition
ChEBI: A polyether that is the dimethyl ether derivative of diethylene glycol.
General Description
Colorless watery liquid with a pleasant odor. Floats and mixes with water.
Air & Water Reactions
Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. A mixture of liquid air and diethyl ether exploded spontaneously, [MCA Case History 616(1960)]. Water soluble.
Reactivity Profile
A violent explosion occurred when lithium aluminum hydride was being used to dry 2-Methoxyethyl ether. The ignition may have occurred due to the presence of large amounts of water or perhaps peroxide formed in the ether. About 75% of the ether had been removed when the explosion occurred, [MCA Case History 1494 (1968)].
Health Hazard
INGESTION (severe cases): nausea, vomiting, abdominal cramps, weakness progressing to coma.
Fire Hazard
2-Methoxyethyl ether is combustible.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Synthesis
265 g (2.5 mol) of diethylene glycol, 320 g (10.0 mol) of methanol, and 14.3 g (0.0125 equivalents) of NAFION 1100 EW Polymer (H+ form) were charged to a one-liter autoclave. After sealing and pressure testing, the contents of the autoclave were agitated, and the autoclave was pressurized to 100 psi with nitrogen. After 5 minutes of agitation, the autoclave was depressurized. This process was repeated two more times to ensure complete deoxygenation. After deoxygenation, the autoclave was heated to a temperature of 198 ℃, and the contents of the autoclave were agitated at 1900 rpm for 5 hours at temperature (198-200 ℃). A pressure of 810 psi was obtained. After 5 hours, the autoclave was cooled and sampled. By analysis, a total of 77.2% by weight of the diethylene glycol (1.93 moles) was converted in the reaction, producing 0.335 mol of Diglyme. The co-product includes 1,4 dioxane and the intermediate diethylene glycol monomethyl ether.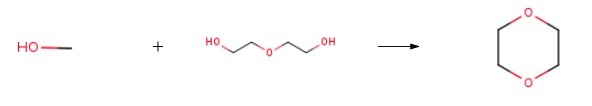
Environmental Fate
The metabolite 2-methoxyacetic acid, which is generated from 2-methroxyethanol by the reaction of alcohol dehydrogenase, may be important for the toxic effects. It can undergo activation to methoxyacetyl coenzyme A and enter the Krebs cycle or fatty acid biosynthesis. Several metabolites of 2-methoxyethanol, such as 2-methoxy-N-acetyl glycine, have been identified that support this pathway. Thus, 2-methoxyacetic acid may interfere with essential metabolic pathways of the cell, and it was hypothesized that this causes the testicular lesions and malformations in experimental animals.
Purification Methods
Dry diglyme with NaOH pellets or CaH2, then reflux with, and distil (under reduced pressure) it from Na, CaH2, LiAlH4, NaBH4 or NaH. These operations are carried out under N2. The amine-like odour of diglyme has been removed by shaking with a weakly acidic ion-exchange resin (Amberlite IR-120) before drying and distilling. Addition of 0.01% NaBH4 to the distillate inhibits peroxidation. Purify it also as for dioxane. It has been passed through a 12-in column of molecular sieves to remove water and peroxides. [Beilstein 1 IV 2393.]
Properties and Applications
Diglyme is also known as Diethylene Glycol Dimethyl Ether. The IUPAC name of this compound is 1-Methoxy-2-(2-methoxyethoxy)ethane. This compound belongs to the class of organic compounds known as dialkyl ethers. These are organic compounds containing the dialkyl ether functional group, with the formula ROR', where R and R' are alkyl groups. In addition, it is an aprotic polar solvent with a high boiling point that is stable at high pH levels. Therefore, it is a suitable solvent for organic reactions conducted under strongly alkaline conditions[1]. Some researchers have already used diglyme as a physical absorbent of CO2.
Toxicity evaluation
Bis (2-methoxyethyl) ether will present as
a vapor, when released to air, at a vapor pressure of
2.96 mmHg at 25°C. The vapor phase is easily degraded in
the atmosphere by reaction with photochemically produced
hydroxyl radicals. The half-life for the reaction is estimated
to be 22 h. Bis (2-methoxyethyl) ether does not contain
chromophores that will absorb at wavelengths >290 nm
and therefore is not expected to be susceptible to direct
photolysis by sunlight.
Bis (2-methoxyethyl) ether has very high
mobility in soil based on the estimated Koc of 15, when released to soil. It may volatilize from dry soil surfaces
based upon its vapor pressure.
Aquatic fate: Bis (2-methoxyethyl) ether does not absorb to
suspended solids and sediments when released into water.
Hydrolysis is not an important environmental matter
because bis (2-methoxyethyl) ether does not contain
a functional group that can hydrolyze under environmental
conditions.
References
[1] Weijia Huang. “An Aprotic Polar Solvent, Diglyme, Combined with Monoethanolamine to Form CO2 Capture Material: Solubility Measurement, Model Correlation, and Effect Evaluation.” Industrial & Engineering Chemistry Research 54 13 (2015): 3430–3437.
Diglyme Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8349 | 58 |
| Anhui lixing chemical co.,ltd | +86-5638152626 +86-17756305689 | gdq@lixingchem.com | China | 10 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | sales@tjzxchem.com | China | 559 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
Related articles
- The role of diethylene glycol dimethy in chemical production
- Diethylene glycol dimethyl ether (DMDE for short) is a colorless neutral liquid with ether odor, boiling point 162 ℃,
- Mar 31,2022
- Uses of Bis (2-methoxyethyl) ether
- Bis (2-methoxyethyl) ether, also known as diglyme, is a linear aliphatic diether widely used as a solvent and present as a cle....
- Nov 25,2021
View Lastest Price from Diglyme manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-27 | DIETHYLENE GLYCOL DIMETHYL ETHER
111-96-6
|
US $0.00-0.00 / kg | 190kg | 99.5%MIN | 3500tons | Anhui lixing chemical co.,ltd | |
 |
2023-08-31 | Diethylene Glycol Dimethyl Ether
111-96-6
|
US $100.00 / bag | 1bag | 99 | 5000 | Hebei Fengqiang Trading Co., LTD | |
 |
2023-07-26 | Diethylene Glycol Dimethyl Ether
111-96-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- DIETHYLENE GLYCOL DIMETHYL ETHER
111-96-6
- US $0.00-0.00 / kg
- 99.5%MIN
- Anhui lixing chemical co.,ltd
-

- Diethylene Glycol Dimethyl Ether
111-96-6
- US $100.00 / bag
- 99
- Hebei Fengqiang Trading Co., LTD
-

- Diethylene Glycol Dimethyl Ether
111-96-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd