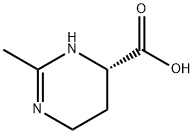
Ectoine
- CAS No.
- 96702-03-3
- Chemical Name:
- Ectoine
- Synonyms
- ECTOIN;Pyrostatine B;RonaCare;ECTOINE BIOCHEMIKA 99%;Ectoine sodium salt monohydrate;(S)-1,4,5,6-Tetrahydro-2-methyl-4-pyrimidincarbonsure;2-METHYL-1,4,5,6-TETRAHYDROPYRIMIDINE-4-CARBOXYLIC ACID;THP[B];Ikdoin;ECTOINE
- CBNumber:
- CB4359836
- Molecular Formula:
- C6H10N2O2
- Molecular Weight:
- 142.16
- MDL Number:
- MFCD03419286
- MOL File:
- 96702-03-3.mol
- MSDS File:
- SDS
| Melting point | ~280° |
|---|---|
| alpha | D20 +140° (c = 1.0 in methanol) |
| Boiling point | 381.5±35.0 °C(Predicted) |
| Density | 1.37 |
| storage temp. | 2-8°C |
| solubility | methanol: 20 mg/mL, clear, colorless |
| pka | 3.14±0.20(Predicted) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Water : 250 mg/mL (1758.58 mM) |
| BRN | 7288977 |
| Stability | Hygroscopic |
| InChI | InChI=1S/C6H10N2O2/c1-4-7-3-2-5(8-4)6(9)10/h5H,2-3H2,1H3,(H,7,8)(H,9,10)/t5-/m0/s1 |
| InChIKey | WQXNXVUDBPYKBA-YFKPBYRVSA-N |
| SMILES | C1(C)=NCC[C@@H](C(O)=O)N1 |
| EWG's Food Scores | 1 |
| FDA UNII | 7GXZ3858RY |
Ectoine price More Price(24)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 81619 | Ectoine ≥95.0% (HPLC) | 96702-03-3 | 1g | $30.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 81619 | Ectoine ≥95.0% (HPLC) | 96702-03-3 | 10g | $247 | 2024-03-01 | Buy |
| Sigma-Aldrich | 81619 | Ectoine ≥95.0% (HPLC) | 96702-03-3 | 100g | $1790 | 2024-03-01 | Buy |
| Sigma-Aldrich | 02380595 | Ectoine primary reference standard | 96702-03-3 | 100MG | $362 | 2024-03-01 | Buy |
| TCI Chemical | E1372 | Ectoine | 96702-03-3 | 1G | $70 | 2024-03-01 | Buy |
Ectoine Chemical Properties,Uses,Production
Description
Ectoine is a carboxamidine heterocycle obtained by formal condensation of (2S)-2,4-diaminobutanoic acid with acetic acid. It has a role as an osmolyte. It is a carboxamidine, a member of 1,4,5,6-tetrahydropyrimidines and a monocarboxylic acid. It is a conjugate acid of an ectoinate. It is a tautomer of an ectoine zwitterion.
a small organic molecule
Ectoine, a small organic molecule, occur widely in aerobic, chemoheterotrophic, and halophilic organisms that enable them to survive under extreme conditions. These organisms protect their biopolymers (bio membranes, proteins, enzymes, and nucleic acids) against dehydration caused by high temperature, salt concentration, and low water activity by substantial ectoine synthesis and enrichment within the cell. The organic osmolyte, ectoine and hydroxyectoine are amphoteric, water-binding, organic molecules. They are generally compatible with the cellular metabolism without adversely affecting the biopolymers or physiologic processes and are so-called compatible solutes.small organic molecule.
Physicochemical properties
Like other compatible solutes, ectoine and 5-hydroxyectoine are low-molecular mass compounds that are highly soluble in water (about 4 M at 20℃), thereby allowing the amassing of these compounds to near molar concentrations in severely osmotically stressed microbial cells. A variety of biophysical techniques have been used to study the effects of ectoine on the hydration of proteins and cell membranes and on interactions mediated via hydrogen bonding. Collectively, these data revealed that ectoine is excluded from the monolayer of dense hydration water around soluble proteins and from the immediate hydration layer at the membrane/liquid interface. Ectoine enhances the properties of hydrogen bonds in aqueous solutions and thereby contributes to the dynamics and stabilization of macromolecular structures. Ectoine possesses a negatively charged carboxylate group attached to a ring structure that contains a delocalized positive charge. The resulting interplay between hydrophilic and hydrophobic forces influences waterwater and water-solute interactions and thereby exerts strong effects on the hydration of ectoine itself, the binding of ions and the influence on the local water structure. Molecular dynamics simulations have indicated that ectoine and 5-hydroxyectoine are strong water-binders and are able to accumulate seven and nine water molecules, respectively, around them at a distance smaller than 0.6 nm. Collectively, the physico-chemical attributes of ectoine allow a physiologically adequate hydration of the cytoplasm upon their osmostress-responsive accumulation, afford effects on the local water structure, and also exert a major protective influence on the stability of proteins and the functionality of macromolecules.
Osmolytic properties of ectoine
Ectoine and 5-hydroxyectoine are produced by microorganisms in response to true osmotic stress, and not just in response to increases in the external salinity. In cases where the build-up of ectoine/5-hydroxyectoine pools has been studied in more detail, there is often a linear relationship between the cellular content of these solutes and the external salinity/osmolarity. This finding implies that bacterial cells can perceive incremental increases in the degree of the environmentally imposed osmotic stress, can process this information genetically/physiologically, and can then set its ectoine/5-hydroxyectoine biosynthetic capacity in a finely tuned fashion to relieve the constraints imposed by high-osmolarity on cellular hydration, physiology, and growth. High-osmolarity-dictated increases in the cellular ectoine pools are largely accomplished through osmotically-responsive increases in the transcription of the ectoine/5-hydroxyectoine biosynthetic genes, although there might be post-transcriptional effects as well. Attesting to the role of ectoine as a potent osmostress protectant is the finding that the disruption of the ectABC biosynthetic genes causes osmotic sensitivity and the genetic disruption of the gene (ectD) for the ectoine hydroxylase in Chromohalobacter salexigens impairs the ability to cope effectively with high growth temperature extremes. In microorganisms that are capable of synthesizing both ectoine and 5-hydroxyectoine, a mixture of these two solutes is frequently found. Interestingly, such a 1:1 mixture (0.5 mM each) provided the best salt and heat stress protection to Streptomyces coelicolor when it was added to the growth medium. However, there are also microorganisms that seem to produce almost exclusively 5-hydroxyectoine during osmotic stress and different growth phases of the culture.
existence
Ectoine is found in high concentrations in halophilic microorganisms and confers resistance towards salt and temperature stress. Ectoine was first identified in the microorganism Ectothiorhodospira halochloris, but has since been found in a wide range of Gram-negative and Gram-positive bacteria.
Biosynthesis of ectoine
Ectoines are common in aerobic heterotrophic eubacteria. The entry molecule in ectoine biosynthesis is aspartate semialdehyde, which is an intermediate in amino acid metabolism. The aldehyde is converted to L-2,4-diaminobutyric acid, which is then acetylated to from Nγ-acetyldiaminobutyric acid (NADA). The final step is the cyclization of this solute to form ectoine. Ectoine synthesis is carried out by the process of three genes: ectABC. The ectA gene codes for diaminobutyric acid acetyltransferase; ectB codes for the diaminobutyric acid aminotransferase and ectC codes for ectoine synthase.
Ectoine is synthesized from aspartate-semialdehyde, the central intermediate in the synthesis of amino acids belonging to the aspartate family. Ectoine formation comprises three enzymatic steps. First, aspartate-semialdehyde is transaminated to 2,4-diaminobutyric acid (DABA) with glutamate as amino-group donor. The transamination is catalyzed by DABA transaminase ectB. EctB is a 421-residue protein with a molecular mass of 46.1 kDa, which requires K 1 for its transaminase activity and for protein stability. Gel filtration experiments with purified protein from H. elongata indicate that the DABA aminotransferase ectB might form a homohexamer in the native state. Then, an acetyl group is transferred to DABA from acetyl-CoA by DABA-Nγ-acetyltransferase ectA in order to synthesize Nγ-acetyl-L-2,4-diaminobutyric acid. EctA is a 192-residue protein with a calculated molecular mass of 21.2 kDa. Finally, ectoine synthase ectC catalyzes the cyclic condensation of Nγ-acetyl-2,4-diaminobutyric acid, which leads to the formation of ectoine. EctC is a 137-residue protein with a calculated molecular weight of 15.5 kDa with a pI value of 4.9. The ectC protein belongs to the enzyme family of carbon-oxygen lyases. In vitro experiments with purified ectC revealed that ectoine-synthase activity and affinity to its substrate are strongly affected by NaCl. Under certain stress conditions (e.g. elevated temperatures) H. elongata converts some of the ectoine to 5- hydroxyectoine by ectoine hydroxylase (EctD). The ectoine hydroxylase EctD consists of 332 amino acids and has a molecular weight of 37.4 kDa. The ectD protein is a member of an oxygenase subfamily within the nonhemecontaining, iron (II)- and α- ketoglutarate-dependent dioxygenase superfamily. Ectoine hydroxylase was shown to catalyze the direct hydroxylation of ectoine to 5-hydroxyectoine. The genes for ectoine biosynthesis ectABC are clustered together and can be found only 5 kb from the termination point of chromosome replication. The ectD gene encoding the hydroxylase for hydroxyectoine synthesis is located apart from the ectABC cluster. Recently, Schwibbert and coworkers mapped the transcriptional initiation sites of ectABC and found two promoters in front of ectA and one upstream of ectC. Upstream of ectA a putative σ70 promoter and an osmotically inducible σ38 promoter were found, while in front of ectC a σ54-controlled promoter is located. σ54-controlled promoters are often involved in transcription of nitrogen-regulated genes. The transcriptional regulation of ectABC by an osmoregulated σ38 promoter and a σ54 promoter is in agreement with physiological observations made with other bacteria, such as Corynebacterium glutamicum and Halorhodospira (formerly Ectothiorhodospira) halochloris. In these organisms, it was shown that synthesis of the compatible solutes proline and glycine betaine, respectively, is not only determined by salinity but also by nitrogen supply. Ectoine biosynthesis is regulated at the level of transcription and enzyme activity. Transcriptome analysis of H. elongata revealed an increase of mRNA from ectA, ectB, and ectC with increasing sodium chloride concentration from 0.6% (0.1 M) to 12% (2 M) NaCl. Similar results were obtained from proteome analysis that showed an increase in ectABC protein at elevated salinities. Contribution of expression and enzyme activity of ectABC to osmoregulated ectoine synthesis was investigated with cells of H. elongata that have been treated with chloramphenicol (Cm) to abolish protein biosynthesis. Cultures of H. elongata with and without Cm growing at 1% NaCl (0.17 M) and 4% NaCl (0.68 M) were exposed to an osmotic upshift. The salt concentration of the cultures growing at 1% NaCl was increased to 4% NaCl and in the cultures growing at 4% NaCl to 8% NaCl. The Cm treated cells grown at a low salt concentration of 1% NaCl failed to synthesize sufficient ectoine in response to the salt shock that raised the salinity to 4% NaCl.
Description
Ectoine is a cyclic tetrahydropyrimidine organic osmolyte, which was discovered in the halophilic bacterium Ectothiorhodospira halochloris. It is the most abundant solute produced by aerobic heterotrophic eubacteria and has been extensively characterized as an osmoprotectant and stabilizer for cells and biomolecules.
Ectoine is considered a member of the small molecule chaperones family (SMCs). SMCs accumulate to high intracellular concentrations, preventing the misfolding/ denaturation of proteins and other labile macromolecules. Ectoine preserves enzymes and whole cells against harmful conditions such as freezing, drying, or heating. This molecule, among other compatible solutes, possesses the ability to conserve the proteolytic activity of trypsin and chymotrypsin. It has been shown to induce heat shock proteins and downregulate proinflammatory signals in human keratinocytes. It is not toxic to the cellular environment, even at concentrations as high as 100 mM.
Ectoine strongly inhibits the Ab42 amyloid formation in vitro, reducing the toxicity to human neurblastoma cells. Ab42 was found to be the most predominant proteolytic fragment found in amyloid plaques, thus, ectoine may act as a potential inhibitor associated with neurodegenerative diseases.
Uses
Ectoine is a natural protector that stabilizes proteins and other cell structures and protects the skin from stresses such as UV exposure and dryness.
Definition
Ectoine is a carboxamidine heterocycle obtained by formal condensation of (2S)-2,4-diaminobutanoic acid with acetic acid. It has a role as an osmolyte. It is a carboxamidine, a member of 1,4,5,6-tetrahydropyrimidines and a monocarboxylic acid. It is a conjugate acid of an ectoinate. It is a tautomer of an ectoine zwitterion.
Biochem/physiol Actions
Ectoine is an osmoprotectant in a variety of microorganisms, including heterotrophic, halophilic and non-halophilic bacteria, such as Streptomyces and E. coli. This compatible solute is also protective for E. coli during drying and storage. Ecdysone is associated with the stabilization of protein and lipid bilayers. It also regulates the retention of bulking pressure and reduces desiccation stress. It can be used in the treatment of inflammatory diseases such as allergic rhinitis, atopic dermatitis and chronic obstructive pulmonary disease. Ecdysone causes structural changes in DNA in vitro and protects DNA from ionizing radiation (IR). It prevents the formation of insulin amyloid in vitro.Ecdysone is an osmoprotectant in a variety of microorganisms, including heterotrophic, halophilic and non-halophilic bacteria, such as Streptomyces and E. coli. This compatible solute is also protective for E. coli during drying and storage. Ecdysone is associated with the stabilization of protein and lipid bilayers. It also regulates the retention of bulking pressure and reduces desiccation stress. It can be used in the treatment of inflammatory diseases such as allergic rhinitis, atopic dermatitis and chronic obstructive pulmonary disease. Ecdysone causes structural changes in DNA in vitro and protects DNA from ionizing radiation (IR). It prevents the formation of insulin amyloid in vitro.
References
1. Galinski, E.A., et al., 1,4,5,6-Tetrahydro-2-methyl-4- pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur. J. Biochem., 149, 135 - 139 (1985).
2. Kanapathipillai, M. et al., Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer’s b-amyloid. FEBS Letters, 579, 4775- 4780 (2005).
3. Kolp, S. et al., Compatible solutes as protectants for zymogens against proteolysis. Biochim. Biophys. Acta, 1764, 1234-1242 (2006).
4. Buommino, E. et al., Ectoine from halophilic microorganisms induces the expression of hsp70 and hsp70B′ in human keratinocytes modulating the proinflammatory response. Cell stress & chap., 10, 197-203 (2005).
Ectoine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Huzhou Haipu Chemical Co.,Ltd | +86-0572-+86-0572-2526078 +8613757061525 | xatyang@acetylacetone.net | China | 16 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 268 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | contact@fuxinpharm.com | China | 10297 | 58 |
| ZhenYiBio Technology Inc | +8615309206328 | alexxue@zhenyibio.com | China | 299 | 58 |
| Chengdu Greenpure Biopharma CO.,Ltd | 18283602253 | jancyzheng@gcgreenpure.com | China | 952 | 58 |
| Maintain Biotech Co., Ltd. | +86-75589664337 +86-15112661142 | cami@maintain-bio.com | China | 66 | 58 |
| Huadong Medicine (Xi'an)Bodyguard Pharmaceutical Co.,Ltd. | +86-029-86185165 +8618629664246 | guoyuan@eastchinapharm.com | China | 1615 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | sales@zhswyy.com | China | 338 | 58 |
| Shanghai Getian Industrial Co., LTD | +undefined15373193816 | mike@ge-tian.com | China | 272 | 58 |
Related articles
- The benefits and uses of Ectoine
- Ectoine as a highly water-keeping compound stabilizing biomolecules and whole cells can be used in scientific work, cosmetics,....
- Nov 3,2023
- What is Ectoine?
- Ectoine shows many beneficial features and has multiple applications. It can contribute in the prevention of stress mediators ....
- Apr 12,2022
- The biosynthesis of Ectoine
- Ectoine is a compatible solute in halophilic organisms.The biosynthesis of ectoine was determined with the help of 13C-labeli....
- Aug 18,2021
Related Qustion
- Q:What is ectoine used for?
- A:Ectoine is a superior moisturizer with long-term efficacy and an active ingredient in skincare and sun protection products.
- Apr 17,2024
View Lastest Price from Ectoine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Ectoine
96702-03-3
|
US $100.00-80.00 / kg | 1kg | 99% | 5000kg/ week | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-04-23 | Ectoine
96702-03-3
|
US $0.00-0.00 / kg | 0.10000000149011612kg | 99% | 2000kgs | Chongqing Zhihe Biopharmaceutical Co., Ltd. | |
 |
2024-04-23 | Tetrahydromethylpyrimidinecarboxylic acid
96702-03-3
|
US $0.00-0.00 / kg | 0.10000000kg | 99% | 2000kg | Chongqing Zhihe Biopharmaceutical Co., Ltd. |
-

- Ectoine
96702-03-3
- US $100.00-80.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Ectoine
96702-03-3
- US $0.00-0.00 / kg
- 99%
- Chongqing Zhihe Biopharmaceutical Co., Ltd.
-

- Tetrahydromethylpyrimidinecarboxylic acid
96702-03-3
- US $0.00-0.00 / kg
- 99%
- Chongqing Zhihe Biopharmaceutical Co., Ltd.
96702-03-3(Ectoine)Related Search:
1of4